当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Benzeneseleninic acid used as an oxidizing and deprotecting reagent for the synthesis of multi-cyclic peptides constrained by multiple disulfide bonds and thioether bridges
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00589a Yueyue Xing 1 , Tianyu Bo 1 , Nan Zhang 1 , Meiqi Wu 1 , Jiawei Wang 1 , Shigang Shen 1 , Yafang Wang 1 , Changying Song 1 , Tiesheng Shi 2 , Shuying Huo 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00589a Yueyue Xing 1 , Tianyu Bo 1 , Nan Zhang 1 , Meiqi Wu 1 , Jiawei Wang 1 , Shigang Shen 1 , Yafang Wang 1 , Changying Song 1 , Tiesheng Shi 2 , Shuying Huo 1
Affiliation
|
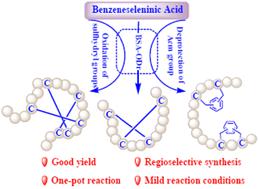
Peptides constrained by multiple disulfide bonds (MDBs) or by thioether bridges play a critical role in improving the biological and pharmaceutical activities of peptide drugs. The synthesis of correct MDBs and thioether-bridged bicyclic peptides still remains a significant challenge. In this work, benzeneseleninic acid (BSA) acting as both an oxidant and a deprotecting reagent for the synthesis of MDB- and thioether-bridged bicyclic peptides has been investigated. Disulfide bonds in peptides can be formed by direct oxidation of two sulfhydryl groups by BSA in neutral media or via two concerted steps, namely deprotecting two acetamidomethyl (Acm) groups and oxidation by BSA in acidic media. As such, two disulfide bonds in α-conotoxin SI, apamin, α-conotoxin IMI and a peptide containing a methionine residue were synthesized regioselectively by the use of the BSA oxidation and deprotection reaction (BSA-ODr). By utilization of the BSA deprotection property, bicyclic peptides were synthesized based on the crosslinking of xylylene dibromide with sulfhydryl groups. Furthermore, two disulfide bonds in α-conotoxin SI and three disulfide bonds in conotoxin mr3e, enterotoxin STp, μ-conotoxin KIIIA, linaclotide and ziconotide were also synthesized regioselectively through oxidation of fully reduced peptides by BSA. All the reactions were carried out under mild conditions in a one-pot manner and peptides with satisfactory yields were achieved. In addition, the BSA-ODr is compatible with methionine residues in the peptides. Moreover, the relative positions of two Acm-protected cysteines and two free cysteines have no impact on the BSA-ODr approach for the construction of two disulfide bonds in peptides. The oxidative folding strategies based on BSA can be executed in peptide manufacture. BSA is readily accessible conferring efficient BSA-ODr and oxidative folding methodologies for the synthesis of MBDs and thioether bridges in peptides.
中文翻译:

苯硒酸用作氧化和脱保护试剂,用于合成受多个二硫键和硫醚桥约束的多环肽
受多个二硫键(MDB)或硫醚桥限制的肽在改善肽药物的生物和药物活性方面发挥着关键作用。正确的 MDB 和硫醚桥双环肽的合成仍然是一个重大挑战。在这项工作中,苯硒酸(BSA)作为氧化剂和脱保护试剂用于MDB桥和硫醚桥双环肽的合成进行了研究。肽中的二硫键可以通过在中性介质中被 BSA 直接氧化两个巯基形成,或者通过两个协同步骤形成,即两个乙酰氨基甲基 (Acm) 基团的脱保护和在酸性介质中被 BSA 氧化。因此,通过使用 BSA 氧化和脱保护反应 (BSA-ODr) 区域选择性地合成了 α-芋螺毒素 SI、apamin、α-芋螺毒素 IMI 和含有甲硫氨酸残基的肽中的两个二硫键。利用BSA的脱保护特性,通过二溴二甲苯与巯基的交联合成了双环肽。此外,还通过BSA氧化完全还原的肽,区域选择性地合成了α-芋螺毒素SI中的两个二硫键和芋螺毒素mr3e、肠毒素STp、μ-芋螺毒素KIIIA、利那洛肽和齐考诺肽中的三个二硫键。所有反应均在温和条件下以一锅法进行,获得了满意收率的肽。此外,BSA-ODr 与肽中的蛋氨酸残基相容。此外,两个 Acm 保护的半胱氨酸和两个游离半胱氨酸的相对位置对构建肽中两个二硫键的 BSA-ODr 方法没有影响。 基于 BSA 的氧化折叠策略可以在肽制造中执行。 BSA 易于获取,可为肽中的 MBD 和硫醚桥的合成提供有效的 BSA-ODr 和氧化折叠方法。
更新日期:2024-06-22
中文翻译:

苯硒酸用作氧化和脱保护试剂,用于合成受多个二硫键和硫醚桥约束的多环肽
受多个二硫键(MDB)或硫醚桥限制的肽在改善肽药物的生物和药物活性方面发挥着关键作用。正确的 MDB 和硫醚桥双环肽的合成仍然是一个重大挑战。在这项工作中,苯硒酸(BSA)作为氧化剂和脱保护试剂用于MDB桥和硫醚桥双环肽的合成进行了研究。肽中的二硫键可以通过在中性介质中被 BSA 直接氧化两个巯基形成,或者通过两个协同步骤形成,即两个乙酰氨基甲基 (Acm) 基团的脱保护和在酸性介质中被 BSA 氧化。因此,通过使用 BSA 氧化和脱保护反应 (BSA-ODr) 区域选择性地合成了 α-芋螺毒素 SI、apamin、α-芋螺毒素 IMI 和含有甲硫氨酸残基的肽中的两个二硫键。利用BSA的脱保护特性,通过二溴二甲苯与巯基的交联合成了双环肽。此外,还通过BSA氧化完全还原的肽,区域选择性地合成了α-芋螺毒素SI中的两个二硫键和芋螺毒素mr3e、肠毒素STp、μ-芋螺毒素KIIIA、利那洛肽和齐考诺肽中的三个二硫键。所有反应均在温和条件下以一锅法进行,获得了满意收率的肽。此外,BSA-ODr 与肽中的蛋氨酸残基相容。此外,两个 Acm 保护的半胱氨酸和两个游离半胱氨酸的相对位置对构建肽中两个二硫键的 BSA-ODr 方法没有影响。 基于 BSA 的氧化折叠策略可以在肽制造中执行。 BSA 易于获取,可为肽中的 MBD 和硫醚桥的合成提供有效的 BSA-ODr 和氧化折叠方法。






































 京公网安备 11010802027423号
京公网安备 11010802027423号