当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lewis acid-catalyzed phosphinoylation and halogenation of α,β-unsaturated ketones: access to γ-halo allylic phosphonates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-17 , DOI: 10.1039/d4qo00748d Xiao-Hong Wei 1 , Ya-Wen Xue 1 , Chun-Yuan Bai 1 , Xuan Liu 1 , Xiao-Hong Wang 1 , Yan-Bin Wang 1 , Qiong Su 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-17 , DOI: 10.1039/d4qo00748d Xiao-Hong Wei 1 , Ya-Wen Xue 1 , Chun-Yuan Bai 1 , Xuan Liu 1 , Xiao-Hong Wang 1 , Yan-Bin Wang 1 , Qiong Su 1
Affiliation
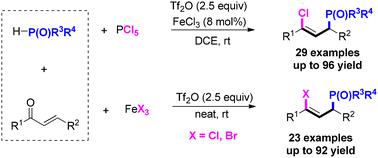
|
A Lewis acid-catalyzed phosphinoylation and halogenation of α,β-unsaturated ketones to synthesize γ-halo allylic phosphonates is described. This transformation consists of a phospha-Michael addition, unimolecular elimination dehydration cascade reaction for the introduction of versatile groups (–P(O)R3R4 and –X) in the resulting γ-substituted allylic phosphonates with high regio- and stereoselectivity.
中文翻译:

路易斯酸催化α,β-不饱和酮的膦酰化和卤化:获得γ-卤代烯丙膦酸酯
描述了路易斯酸催化 α,β-不饱和酮的膦酰化和卤化来合成 γ-卤代烯丙膦酸酯。该转化包括磷酸-迈克尔加成、单分子消除脱水级联反应,以在所得产物中引入多功能基团(-P(O)R 3 R 4 和 -X)具有高区域选择性和立体选择性的γ-取代烯丙基膦酸酯。
更新日期:2024-06-20
中文翻译:

路易斯酸催化α,β-不饱和酮的膦酰化和卤化:获得γ-卤代烯丙膦酸酯
描述了路易斯酸催化 α,β-不饱和酮的膦酰化和卤化来合成 γ-卤代烯丙膦酸酯。该转化包括磷酸-迈克尔加成、单分子消除脱水级联反应,以在所得产物中引入多功能基团(-P(O)R 3 R 4 和 -X)具有高区域选择性和立体选择性的γ-取代烯丙基膦酸酯。






































 京公网安备 11010802027423号
京公网安备 11010802027423号