当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Brønsted acid-mediated selective α-alkenylation of 3,4-dihydro-2H-pyrans
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-17 , DOI: 10.1039/d4qo00541d Nan Chen 1 , Chaokun Li 1 , Shangteng Liao 2 , Jinglong Chen 2 , Xingxing Ma 1 , Qiuling Song 1, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-17 , DOI: 10.1039/d4qo00541d Nan Chen 1 , Chaokun Li 1 , Shangteng Liao 2 , Jinglong Chen 2 , Xingxing Ma 1 , Qiuling Song 1, 3, 4
Affiliation
|
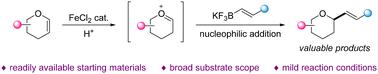
A protocol for the synthesis of α-alkenylated tetrahydropyrans (THPs) with 3,4-dihydro-2H-pyran derivatives and potassium alkenyltrifluoroborates was achieved in the presence of a Brønsted acid and a Lewis acid under mild reaction conditions. The oxonium ion intermediates, produced from various readily available 3,4-dihydro-2H-pyran derivatives, were attacked by the nucleophile to form the desired α-alkenylated products. This strategy could be an alternative approach to modify a series of modules that are of broad utility in natural products and fine chemicals.
中文翻译:

布朗斯台德酸介导的 3,4-二氢-2H-吡喃的选择性 α-烯基化
在布朗斯台德酸和路易斯酸存在下,在温和的反应条件下,实现了用 3,4-二氢-2H-吡喃衍生物和烯基三氟硼酸钾合成 α-烯基化四氢吡喃 (THP) 的方案。由各种容易获得的 3,4-二氢-2H-吡喃衍生物产生的氧鎓离子中间体被亲核试剂攻击,形成所需的 α-烯基化产物。该策略可能是修改一系列在天然产品和精细化学品中广泛应用的模块的替代方法。
更新日期:2024-06-21
中文翻译:

布朗斯台德酸介导的 3,4-二氢-2H-吡喃的选择性 α-烯基化
在布朗斯台德酸和路易斯酸存在下,在温和的反应条件下,实现了用 3,4-二氢-2H-吡喃衍生物和烯基三氟硼酸钾合成 α-烯基化四氢吡喃 (THP) 的方案。由各种容易获得的 3,4-二氢-2H-吡喃衍生物产生的氧鎓离子中间体被亲核试剂攻击,形成所需的 α-烯基化产物。该策略可能是修改一系列在天然产品和精细化学品中广泛应用的模块的替代方法。






































 京公网安备 11010802027423号
京公网安备 11010802027423号