当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Antifungal Agent Voriconazole via Asymmetric Epoxidation of Tetrasubstituted (Z)-Alkene
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-06-14 , DOI: 10.1002/ejoc.202400400 Jiale Peng 1 , Ran Li 1 , Zhuo Li 1 , Yu Tang 1 , Jin‐Song Yang 1 , Fei Xue 1 , Yong Qin 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-06-14 , DOI: 10.1002/ejoc.202400400 Jiale Peng 1 , Ran Li 1 , Zhuo Li 1 , Yu Tang 1 , Jin‐Song Yang 1 , Fei Xue 1 , Yong Qin 1
Affiliation
|
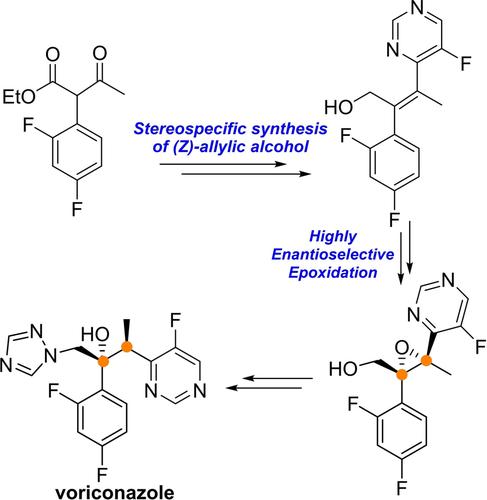
The catalytic asymmetric synthesis of voriconazole, a synthetically challenging antifungal agent was achieved in nine steps with a 8 % overall yield. This novel strategy relied on the precise synthesis of tetrasubstituted (Z)-allylic alcohol and the subsequent highly efficient epoxidation to establish the critical contiguous carbon stereocenters in a single step.
中文翻译:

四取代(Z)-烯烃不对称环氧化对映选择性合成抗真菌药伏立康唑
伏立康唑(一种合成上具有挑战性的抗真菌剂)的催化不对称合成通过九个步骤实现,总产率为 8%。这种新颖的策略依赖于四取代(Z)-烯丙醇的精确合成以及随后的高效环氧化,以一步建立关键的连续碳立构中心。
更新日期:2024-06-17
中文翻译:

四取代(Z)-烯烃不对称环氧化对映选择性合成抗真菌药伏立康唑
伏立康唑(一种合成上具有挑战性的抗真菌剂)的催化不对称合成通过九个步骤实现,总产率为 8%。这种新颖的策略依赖于四取代(Z)-烯丙醇的精确合成以及随后的高效环氧化,以一步建立关键的连续碳立构中心。






































 京公网安备 11010802027423号
京公网安备 11010802027423号