当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selectivity control by modifying pressure, temperature, and ionomer decoration for CO2 electroreduction using gas-diffusion Cu electrodes
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-06-17 , DOI: 10.1016/j.checat.2024.101030
Xiaofei Lu , Tengisbold Gankhuyag , Keisuke Obata , Yuhang Yu , Kazuhiro Takanabe
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-06-17 , DOI: 10.1016/j.checat.2024.101030
Xiaofei Lu , Tengisbold Gankhuyag , Keisuke Obata , Yuhang Yu , Kazuhiro Takanabe
|
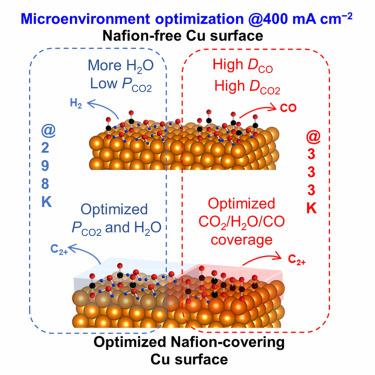
“Beyond catalyst” factors influencing the performance of Cu-based CO electroreduction were investigated based on rigorous microkinetic analysis under industrially relevant conditions. Our experimental results indicated that the activity and selectivity of Cu-based CO electroreduction were not unaffected by electrolyte pH changes but were significantly influenced by operating conditions including pressure and temperature. Analyzing the kinetic data concerning CO partial pressure () and operating temperature revealed that higher (≥25 kPa) or higher reaction temperature (≤333 K) favored CO formation, coinciding with a reduction of the C and formate pathways. Product distribution control at 333 K was achieved by engineering the structure of the Cu gas-diffusion electrode (GDE), where CO was exclusively produced on bare Cu, and the faradic efficiency of C was enhanced by introducing an optimized Nafion ionomer content in the catalyst layer, likely ascribable to the modulation of diffusion coefficients of reactants (e.g., CO and HO) and key intermediates (e.g., CO).
中文翻译:

通过改变压力、温度和离聚物修饰来控制使用气体扩散铜电极进行 CO2 电解的选择性
基于工业相关条件下严格的微动力学分析,研究了影响铜基 CO 电还原性能的“催化剂之外”因素。我们的实验结果表明,铜基CO电还原的活性和选择性并非不受电解质pH变化的影响,而是受到压力和温度等操作条件的显着影响。分析有关 CO 分压 () 和操作温度的动力学数据表明,较高 (≥25 kPa) 或较高反应温度 (≤333 K) 有利于 CO 形成,与 C 和甲酸盐途径的减少相一致。通过设计 Cu 气体扩散电极 (GDE) 的结构实现了 333 K 的产物分布控制,其中 CO 完全在裸铜上产生,并且通过在催化剂中引入优化的 Nafion 离聚物含量来提高 C 的法拉第效率层,可能归因于反应物(例如 CO 和 H2O)和关键中间体(例如 CO)扩散系数的调节。
更新日期:2024-06-17
中文翻译:

通过改变压力、温度和离聚物修饰来控制使用气体扩散铜电极进行 CO2 电解的选择性
基于工业相关条件下严格的微动力学分析,研究了影响铜基 CO 电还原性能的“催化剂之外”因素。我们的实验结果表明,铜基CO电还原的活性和选择性并非不受电解质pH变化的影响,而是受到压力和温度等操作条件的显着影响。分析有关 CO 分压 () 和操作温度的动力学数据表明,较高 (≥25 kPa) 或较高反应温度 (≤333 K) 有利于 CO 形成,与 C 和甲酸盐途径的减少相一致。通过设计 Cu 气体扩散电极 (GDE) 的结构实现了 333 K 的产物分布控制,其中 CO 完全在裸铜上产生,并且通过在催化剂中引入优化的 Nafion 离聚物含量来提高 C 的法拉第效率层,可能归因于反应物(例如 CO 和 H2O)和关键中间体(例如 CO)扩散系数的调节。































 京公网安备 11010802027423号
京公网安备 11010802027423号