当前位置:
X-MOL 学术
›
Am. J. Hematol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roxadustat versus placebo for patients with lower-risk myelodysplastic syndrome: MATTERHORN phase 3, double-blind, randomized controlled trial
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-06-17 , DOI: 10.1002/ajh.27410 Moshe Mittelman 1 , David H Henry 2 , John A Glaspy 3 , Anil Tombak 4 , Rosemary Harrup 5 , Inho Kim 6 , Krzysztof Mądry 7 , Barbara Grabowska 8 , Tyson Lee 9 , Katharina Modelska 9
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-06-17 , DOI: 10.1002/ajh.27410 Moshe Mittelman 1 , David H Henry 2 , John A Glaspy 3 , Anil Tombak 4 , Rosemary Harrup 5 , Inho Kim 6 , Krzysztof Mądry 7 , Barbara Grabowska 8 , Tyson Lee 9 , Katharina Modelska 9
Affiliation
|
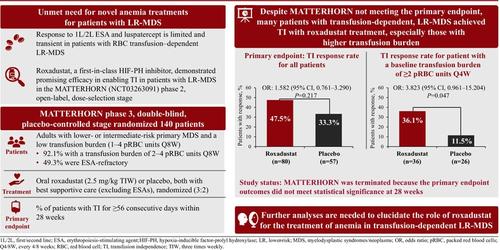
In patients with lower-risk myelodysplastic syndromes/neoplasms (MDS), response to first-line therapy is limited and transient. The MATTERHORN randomized, double-blind, phase 3 trial evaluated roxadustat versus placebo for patients with transfusion-dependent, lower-risk MDS. Eligible patients had very low-, low-, or intermediate-risk MDS with or without prior erythropoiesis-stimulating agent treatment, and a transfusion burden of 1–4 packed red blood cell (pRBC) units every 8 weeks (Q8W). Patients were randomized (3:2) to oral roxadustat (2.5 mg/kg) or placebo, both three times weekly, with best supportive care. Primary efficacy endpoint was transfusion independence (TI) for ≥56 days within 28 weeks (TI responders). MATTERHORN was terminated due to interim analysis outcomes not meeting statistical significance. In total, 272 patients were screened, and 140 patients were enrolled (82, roxadustat, and 58, placebo). At final analysis, 38/80 (47.5%) patients and 19/57 (33.3%) in the roxadustat and placebo arms, respectively, were TI responders (p = .217). A greater percentage of patients in the roxadustat arm with a transfusion burden of ≥2 pRBC units Q4W were TI responders (36.1%; 13/36) compared with the placebo arm (11.5%; 3/26; p-nominal = .047). The seven on-study deaths (4, roxadustat, and 3, placebo) were considered unrelated to treatment. Three roxadustat patients progressed to acute myeloid leukemia. Despite MATTERHORN not meeting its primary endpoint, a numerically higher TI rate was achieved with roxadustat treatment compared with placebo. Further analyses are needed to confirm the MDS patient subgroups deriving clinical benefit from this novel treatment.
中文翻译:

Roxadustat 与安慰剂治疗低风险骨髓增生异常综合征患者的比较:MATTERHORN 3 期、双盲、随机对照试验
对于低危骨髓增生异常综合征/肿瘤 (MDS) 患者,一线治疗的反应有限且短暂。 MATTERHORN 随机、双盲、3 期试验评估了 roxadustat 与安慰剂对输血依赖性、低风险 MDS 患者的疗效。符合条件的患者患有极低、低或中等风险的MDS,无论是否接受过红细胞生成刺激剂治疗,并且每8周(Q8W)输注1-4个浓缩红细胞(pRBC)单位。患者被随机 (3:2) 接受口服罗沙司他 (2.5 mg/kg) 或安慰剂治疗,每周 3 次,并接受最佳支持治疗。主要疗效终点是 28 周内输血独立性 (TI) ≥ 56 天(TI 反应者)。由于中期分析结果不符合统计显着性,马特洪峰被终止。总共筛选了 272 名患者,并入组了 140 名患者(82 名患者为罗沙司他,58 名患者为安慰剂)。最终分析显示,roxadustat 组和安慰剂组分别有 38/80 (47.5%) 例患者和 19/57 (33.3%) 例患者为 TI 缓解者 ( p = .217)。与安慰剂组 (11.5%; 3/26; p标称值 = .047) 相比,罗沙司他组中 Q4W 输血负担≥2 pRBC 单位的患者中 TI 应答者的比例更高 (36.1%; 13/36) (36.1%; 13/36) 。研究中的 7 例死亡(4 例为罗沙司他,3 例为安慰剂)被认为与治疗无关。三名罗沙司他患者进展为急性髓系白血病。尽管 MATTERHORN 未达到其主要终点,但与安慰剂相比,罗沙司他治疗的 TI 率在数值上更高。需要进一步分析来确认 MDS 患者亚组从这种新疗法中获得临床获益。
更新日期:2024-06-17
中文翻译:

Roxadustat 与安慰剂治疗低风险骨髓增生异常综合征患者的比较:MATTERHORN 3 期、双盲、随机对照试验
对于低危骨髓增生异常综合征/肿瘤 (MDS) 患者,一线治疗的反应有限且短暂。 MATTERHORN 随机、双盲、3 期试验评估了 roxadustat 与安慰剂对输血依赖性、低风险 MDS 患者的疗效。符合条件的患者患有极低、低或中等风险的MDS,无论是否接受过红细胞生成刺激剂治疗,并且每8周(Q8W)输注1-4个浓缩红细胞(pRBC)单位。患者被随机 (3:2) 接受口服罗沙司他 (2.5 mg/kg) 或安慰剂治疗,每周 3 次,并接受最佳支持治疗。主要疗效终点是 28 周内输血独立性 (TI) ≥ 56 天(TI 反应者)。由于中期分析结果不符合统计显着性,马特洪峰被终止。总共筛选了 272 名患者,并入组了 140 名患者(82 名患者为罗沙司他,58 名患者为安慰剂)。最终分析显示,roxadustat 组和安慰剂组分别有 38/80 (47.5%) 例患者和 19/57 (33.3%) 例患者为 TI 缓解者 ( p = .217)。与安慰剂组 (11.5%; 3/26; p标称值 = .047) 相比,罗沙司他组中 Q4W 输血负担≥2 pRBC 单位的患者中 TI 应答者的比例更高 (36.1%; 13/36) (36.1%; 13/36) 。研究中的 7 例死亡(4 例为罗沙司他,3 例为安慰剂)被认为与治疗无关。三名罗沙司他患者进展为急性髓系白血病。尽管 MATTERHORN 未达到其主要终点,但与安慰剂相比,罗沙司他治疗的 TI 率在数值上更高。需要进一步分析来确认 MDS 患者亚组从这种新疗法中获得临床获益。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号