当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mitochondria-targeted polyprodrug nanoparticles induce mitochondrial stress for immunogenic chemo-photodynamic therapy of ovarian cancer
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-10 , DOI: 10.1016/j.jconrel.2024.06.014 Wenjia Zhang 1 , Gui Chen 1 , Ziqi Chen 2 , Xin Yang 3 , Bingchen Zhang 1 , Shengtao Wang 4 , Zibo Li 1 , Yuanyuan Yang 1 , Yifen Wu 5 , Zhigang Liu 6 , Zhiqiang Yu 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-10 , DOI: 10.1016/j.jconrel.2024.06.014 Wenjia Zhang 1 , Gui Chen 1 , Ziqi Chen 2 , Xin Yang 3 , Bingchen Zhang 1 , Shengtao Wang 4 , Zibo Li 1 , Yuanyuan Yang 1 , Yifen Wu 5 , Zhigang Liu 6 , Zhiqiang Yu 1
Affiliation
|
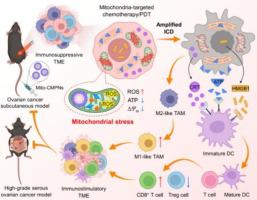
Hypoimmunogenicity and the immunosuppressive microenvironment of ovarian cancer severely restrict the capability of immune-mediated tumor killing. Immunogenic cell death (ICD) introduces a theoretical principle for antitumor immunity by increasing antigen exposure and presentation. Despite recent research progress, the currently available ICD inducers are still very limited, and many of them can hardly induce sufficient ICD based on traditional endoplasmic reticulum (ER) stress. Accumulating evidence indicates that inducing mitochondrial stress usually shows a higher efficiency in evoking large-scale ICD than that ER stress. Inspired by this, herein, a mitochondria-targeted polyprodrug nanoparticle (named Mito-CMPN) serves as a much superior ICD inducer, effectively inducing chemo-photodynamic therapy-caused mitochondrial stress in tumor cells. The rationally designed stimuli-responsive polyprodrugs, which can self-assemble into nanoparticles, were functionalized with rhodamine B for mitochondrial targeting, cisplatin and mitoxantrone (MTO) for synergistic chemo-immunotherapy, and MTO also serves as a photosensitizer for photodynamic immunotherapy. The effectiveness and robustness of Mito-CMPNs in reversing the immunosuppressive microenvironment is verified in both an ovarian cancer subcutaneous model and a high-grade serous ovarian cancer model. Our results support that the induction of abundant ICD by focused mitochondrial stress is a highly effective strategy to improve the therapeutic efficacy of immunosuppressive ovarian cancer.
中文翻译:

线粒体靶向多聚前药纳米颗粒诱导线粒体应激,用于卵巢癌的免疫原性化学光动力治疗
卵巢癌的低免疫原性和免疫抑制微环境严重限制了免疫介导的肿瘤杀伤能力。免疫原性细胞死亡(ICD)通过增加抗原暴露和呈递引入了抗肿瘤免疫的理论原理。尽管最近的研究取得了进展,但目前可用的 ICD 诱导剂仍然非常有限,其中许多很难基于传统的内质网(ER)应激诱导足够的 ICD。越来越多的证据表明,诱导线粒体应激通常比内质网应激更能有效地引发大规模 ICD。受此启发,本文中,一种靶向线粒体的聚前药纳米颗粒(名为 Mito-CMPN)作为一种优越的 ICD 诱导剂,有效诱导肿瘤细胞中化学光动力疗法引起的线粒体应激。合理设计的刺激响应性聚前药可以自组装成纳米颗粒,并用罗丹明 B 进行功能化,用于线粒体靶向,顺铂和米托蒽醌 (MTO) 进行协同化学免疫治疗,MTO 还可以作为光动力免疫治疗的光敏剂。 Mito-CMPNs 在逆转免疫抑制微环境方面的有效性和稳健性在卵巢癌皮下模型和高级别浆液性卵巢癌模型中得到验证。我们的结果表明,通过集中线粒体应激诱导大量 ICD 是提高免疫抑制性卵巢癌治疗效果的高效策略。
更新日期:2024-06-10
中文翻译:

线粒体靶向多聚前药纳米颗粒诱导线粒体应激,用于卵巢癌的免疫原性化学光动力治疗
卵巢癌的低免疫原性和免疫抑制微环境严重限制了免疫介导的肿瘤杀伤能力。免疫原性细胞死亡(ICD)通过增加抗原暴露和呈递引入了抗肿瘤免疫的理论原理。尽管最近的研究取得了进展,但目前可用的 ICD 诱导剂仍然非常有限,其中许多很难基于传统的内质网(ER)应激诱导足够的 ICD。越来越多的证据表明,诱导线粒体应激通常比内质网应激更能有效地引发大规模 ICD。受此启发,本文中,一种靶向线粒体的聚前药纳米颗粒(名为 Mito-CMPN)作为一种优越的 ICD 诱导剂,有效诱导肿瘤细胞中化学光动力疗法引起的线粒体应激。合理设计的刺激响应性聚前药可以自组装成纳米颗粒,并用罗丹明 B 进行功能化,用于线粒体靶向,顺铂和米托蒽醌 (MTO) 进行协同化学免疫治疗,MTO 还可以作为光动力免疫治疗的光敏剂。 Mito-CMPNs 在逆转免疫抑制微环境方面的有效性和稳健性在卵巢癌皮下模型和高级别浆液性卵巢癌模型中得到验证。我们的结果表明,通过集中线粒体应激诱导大量 ICD 是提高免疫抑制性卵巢癌治疗效果的高效策略。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号