当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delivery of nucleic acid based genome editing platforms via lipid nanoparticles: Clinical applications
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.addr.2024.115359 Razan Masarwy 1 , Lior Stotsky-Oterin 2 , Aviad Elisha 1 , Inbal Hazan-Halevy 2 , Dan Peer 2
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.addr.2024.115359 Razan Masarwy 1 , Lior Stotsky-Oterin 2 , Aviad Elisha 1 , Inbal Hazan-Halevy 2 , Dan Peer 2
Affiliation
|
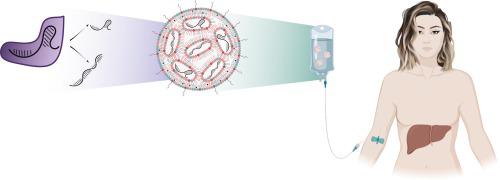
CRISPR/Cas technology presents a promising approach for treating a wide range of diseases, including cancer and genetic disorders. Despite its potential, the translation of CRISPR/Cas into effective in-vivo gene therapy encounters challenges, primarily due to the need for safe and efficient delivery mechanisms. Lipid nanoparticles (LNPs), FDA-approved for RNA delivery, show potential for delivering also CRISPR/Cas, offering the capability to efficiently encapsulate large mRNA molecules with single guide RNAs. However, achieving precise targeting in-vivo remains a significant obstacle, necessitating further research into optimizing LNP formulations. Strategies to enhance specificity, such as modifying LNP structures and incorporating targeting ligands, are explored to improve organ and cell type targeting. Furthermore, the development of base and prime editing technology presents a potential breakthrough, offering precise modifications without generating double-strand breaks (DSBs). Prime editing, particularly when delivered via targeted LNPs, holds promise for treating diverse diseases safely and precisely. This review assesses both the progress made and the persistent challenges faced in using LNP-encapsulated CRISPR-based technologies for therapeutic purposes, with a particular focus on clinical translation.
中文翻译:

通过脂质纳米粒子提供基于核酸的基因组编辑平台:临床应用
CRISPR/Cas 技术为治疗包括癌症和遗传性疾病在内的多种疾病提供了一种有前途的方法。尽管具有潜力,但将 CRISPR/Cas 转化为有效的体内基因疗法仍面临挑战,这主要是由于需要安全有效的递送机制。 FDA 批准用于 RNA 递送的脂质纳米粒子 (LNP) 也显示出递送 CRISPR/Cas 的潜力,能够用单引导 RNA 有效封装大 mRNA 分子。然而,实现体内精确靶向仍然是一个重大障碍,需要进一步研究优化 LNP 配方。探索增强特异性的策略,例如修改 LNP 结构和合并靶向配体,以改善器官和细胞类型靶向。此外,碱基和引物编辑技术的发展带来了潜在的突破,可以提供精确的修饰而不产生双链断裂(DSB)。 Prime 编辑,特别是通过靶向 LNP 进行编辑时,有望安全、精确地治疗多种疾病。本综述评估了使用 LNP 封装的 CRISPR 技术用于治疗目的所取得的进展和面临的持续挑战,特别关注临床转化。
更新日期:2024-06-08
中文翻译:

通过脂质纳米粒子提供基于核酸的基因组编辑平台:临床应用
CRISPR/Cas 技术为治疗包括癌症和遗传性疾病在内的多种疾病提供了一种有前途的方法。尽管具有潜力,但将 CRISPR/Cas 转化为有效的体内基因疗法仍面临挑战,这主要是由于需要安全有效的递送机制。 FDA 批准用于 RNA 递送的脂质纳米粒子 (LNP) 也显示出递送 CRISPR/Cas 的潜力,能够用单引导 RNA 有效封装大 mRNA 分子。然而,实现体内精确靶向仍然是一个重大障碍,需要进一步研究优化 LNP 配方。探索增强特异性的策略,例如修改 LNP 结构和合并靶向配体,以改善器官和细胞类型靶向。此外,碱基和引物编辑技术的发展带来了潜在的突破,可以提供精确的修饰而不产生双链断裂(DSB)。 Prime 编辑,特别是通过靶向 LNP 进行编辑时,有望安全、精确地治疗多种疾病。本综述评估了使用 LNP 封装的 CRISPR 技术用于治疗目的所取得的进展和面临的持续挑战,特别关注临床转化。































 京公网安备 11010802027423号
京公网安备 11010802027423号