当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advances, challenges, and future directions in the clinical translation of ECM biomaterials for regenerative medicine applications
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-04 , DOI: 10.1016/j.addr.2024.115347 Héctor Capella-Monsonís 1 , Raphael J Crum 2 , George S Hussey 3 , Stephen F Badylak 4
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-04 , DOI: 10.1016/j.addr.2024.115347 Héctor Capella-Monsonís 1 , Raphael J Crum 2 , George S Hussey 3 , Stephen F Badylak 4
Affiliation
|
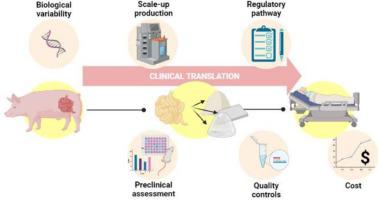
Extracellular Matrix (ECM) scaffolds and biomaterials have been widely used for decades across a variety of diverse clinical applications and have been implanted in millions of patients worldwide. ECM-based biomaterials have been especially successful in soft tissue repair applications but their utility in other clinical applications such as for regeneration of bone or neural tissue is less well understood. The beneficial healing outcome with the use of ECM biomaterials is the result of their biocompatibility, their biophysical properties and their ability to modify cell behavior after injury. As a consequence of successful clinical outcomes, there has been motivation for the development of next-generation formulations of ECM materials ranging from hydrogels, bioinks, powders, to whole organ or tissue scaffolds. The continued development of novel ECM formulations as well as active research interest in these materials ensures a wealth of possibilities for future clinical translation and innovation in regenerative medicine. The clinical translation of next generation formulations ECM scaffolds faces predictable challenges such as manufacturing, manageable regulatory pathways, surgical implantation, and the cost required to address these challenges. The current status of ECM-based biomaterials, including clinical translation, novel formulations and therapies currently under development, and the challenges that limit clinical translation of ECM biomaterials are reviewed herein.
中文翻译:

用于再生医学应用的 ECM 生物材料临床转化的进展、挑战和未来方向
细胞外基质 (ECM) 支架和生物材料几十年来已在各种不同的临床应用中广泛使用,并已植入全球数百万患者体内。基于 ECM 的生物材料在软组织修复应用中特别成功,但其在其他临床应用(例如骨或神经组织再生)中的效用尚不清楚。使用 ECM 生物材料的有益愈合效果是其生物相容性、生物物理特性以及损伤后改变细胞行为的能力的结果。由于成功的临床结果,人们有动力开发下一代 ECM 材料配方,从水凝胶、生物墨水、粉末到整个器官或组织支架。新型 ECM 配方的持续开发以及对这些材料的积极研究兴趣确保了再生医学未来临床转化和创新的丰富可能性。下一代配方 ECM 支架的临床转化面临着可预见的挑战,例如制造、可管理的监管途径、手术植入以及应对这些挑战所需的成本。本文综述了基于 ECM 的生物材料的现状,包括临床转化、目前正在开发的新制剂和疗法,以及限制 ECM 生物材料临床转化的挑战。
更新日期:2024-06-04
中文翻译:

用于再生医学应用的 ECM 生物材料临床转化的进展、挑战和未来方向
细胞外基质 (ECM) 支架和生物材料几十年来已在各种不同的临床应用中广泛使用,并已植入全球数百万患者体内。基于 ECM 的生物材料在软组织修复应用中特别成功,但其在其他临床应用(例如骨或神经组织再生)中的效用尚不清楚。使用 ECM 生物材料的有益愈合效果是其生物相容性、生物物理特性以及损伤后改变细胞行为的能力的结果。由于成功的临床结果,人们有动力开发下一代 ECM 材料配方,从水凝胶、生物墨水、粉末到整个器官或组织支架。新型 ECM 配方的持续开发以及对这些材料的积极研究兴趣确保了再生医学未来临床转化和创新的丰富可能性。下一代配方 ECM 支架的临床转化面临着可预见的挑战,例如制造、可管理的监管途径、手术植入以及应对这些挑战所需的成本。本文综述了基于 ECM 的生物材料的现状,包括临床转化、目前正在开发的新制剂和疗法,以及限制 ECM 生物材料临床转化的挑战。






























 京公网安备 11010802027423号
京公网安备 11010802027423号