当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A comprehensive comparison of DNA and RNA vaccines
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-05-27 , DOI: 10.1016/j.addr.2024.115340 Chunxi Wang 1 , Fan Yuan 1
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-05-27 , DOI: 10.1016/j.addr.2024.115340 Chunxi Wang 1 , Fan Yuan 1
Affiliation
|
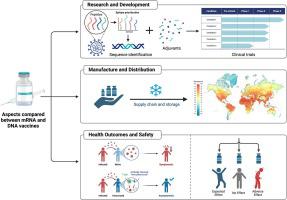
Nucleic acid technology has revolutionized vaccine development, enabling rapid design and production of RNA and DNA vaccines for prevention and treatment of diseases. The successful deployment of mRNA and plasmid DNA vaccines against COVID-19 has further validated the technology. At present, mRNA platform is prevailing due to its higher efficacy, while DNA platform is undergoing rapid evolution because it possesses unique advantages that can potentially overcome the problems associated with the mRNA platform. To help understand the recent performances of the two vaccine platforms and recognize their clinical potentials in the future, this review compares the advantages and drawbacks of mRNA and DNA vaccines that are currently known in the literature, in terms of development timeline, financial cost, ease of distribution, efficacy, safety, and regulatory approval of products. Additionally, the review discusses the ongoing clinical trials, strategies for improvement, and alternative designs of RNA and DNA platforms for vaccination.
中文翻译:

DNA和RNA疫苗的全面比较
核酸技术彻底改变了疫苗开发,能够快速设计和生产用于预防和治疗疾病的 RNA 和 DNA 疫苗。针对 COVID-19 的 mRNA 和质粒 DNA 疫苗的成功部署进一步验证了该技术。目前,mRNA平台因其更高的功效而盛行,而DNA平台正在快速进化,因为它拥有独特的优势,有可能克服mRNA平台的相关问题。为了帮助了解这两个疫苗平台的近期表现并认识它们未来的临床潜力,本综述比较了目前文献中已知的 mRNA 和 DNA 疫苗在开发时间表、财务成本、易用性方面的优缺点。产品的分销、功效、安全性和监管批准。此外,该综述还讨论了正在进行的临床试验、改进策略以及用于疫苗接种的 RNA 和 DNA 平台的替代设计。
更新日期:2024-05-27
中文翻译:

DNA和RNA疫苗的全面比较
核酸技术彻底改变了疫苗开发,能够快速设计和生产用于预防和治疗疾病的 RNA 和 DNA 疫苗。针对 COVID-19 的 mRNA 和质粒 DNA 疫苗的成功部署进一步验证了该技术。目前,mRNA平台因其更高的功效而盛行,而DNA平台正在快速进化,因为它拥有独特的优势,有可能克服mRNA平台的相关问题。为了帮助了解这两个疫苗平台的近期表现并认识它们未来的临床潜力,本综述比较了目前文献中已知的 mRNA 和 DNA 疫苗在开发时间表、财务成本、易用性方面的优缺点。产品的分销、功效、安全性和监管批准。此外,该综述还讨论了正在进行的临床试验、改进策略以及用于疫苗接种的 RNA 和 DNA 平台的替代设计。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号