当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dysfunction of astrocytic glycophagy exacerbates reperfusion injury in ischemic stroke
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-07 , DOI: 10.1016/j.redox.2024.103234 Haiyun Guo , Yumeng Li , Shiquan Wang , Yongheng Yang , Tiantian Xu , Jianshuai Zhao , Jin Wang , Wenqiang Zuo , Pengju Wang , Guangchao Zhao , Huaning Wang , Wugang Hou , Hailong Dong , Yanhui Cai
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-07 , DOI: 10.1016/j.redox.2024.103234 Haiyun Guo , Yumeng Li , Shiquan Wang , Yongheng Yang , Tiantian Xu , Jianshuai Zhao , Jin Wang , Wenqiang Zuo , Pengju Wang , Guangchao Zhao , Huaning Wang , Wugang Hou , Hailong Dong , Yanhui Cai
|
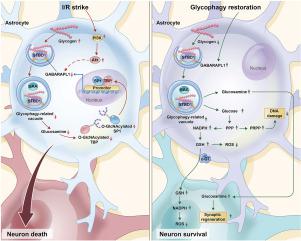
Glycophagy has evolved from an alternative glycogen degradation pathway into a multifaceted pivot to regulate cellular metabolic hemostasis in peripheral tissues. However, the pattern of glycophagy in the brain and its potential therapeutic impact on ischemic stroke remain unknown. Here, we observed that the dysfunction of astrocytic glycophagy was caused by the downregulation of the GABA type A receptor-associated protein like 1 (GABARAPL1) during reperfusion in ischemic stroke patients and mice. PI3K-Akt pathway activation is involved in driving GABARAPL1 downregulation during cerebral reperfusion. Moreover, glycophagy dysfunction-induced glucosamine deficiency suppresses the nuclear translocation of specificity protein 1 and TATA binding protein, the transcription factors for GABARAPL1, by decreasing their O-GlcNAcylation levels, and accordingly feedback inhibits GABARAPL1 in astrocytes during reperfusion. Restoring astrocytic glycophagy by overexpressing GABARAPL1 decreases DNA damage and oxidative injury in astrocytes and improves the survival of surrounding neurons during reperfusion. In addition, a hypocaloric diet in the acute phase after cerebral reperfusion can enhance astrocytic glycophagic flux and accelerate neurological recovery. In summary, glycophagy in the brain links autophagy, metabolism, and epigenetics together, and glycophagy dysfunction exacerbates reperfusion injury after ischemic stroke.
中文翻译:

星形细胞糖吞噬功能障碍加剧缺血性中风的再灌注损伤
糖吞噬已从一种替代的糖原降解途径演变为调节外周组织细胞代谢止血的多方面枢纽。然而,大脑中的糖吞噬模式及其对缺血性中风的潜在治疗作用仍然未知。在这里,我们观察到缺血性中风患者和小鼠再灌注期间星形胶质细胞糖吞噬功能障碍是由 GABA A 型受体相关蛋白样 1 (GABARAPL1) 的下调引起的。 PI3K-Akt 通路激活参与脑再灌注期间 GABARAPL1 下调。此外,糖吞噬功能障碍诱导的葡萄糖胺缺乏通过降低 GABARAPL1 转录因子 GABARAPL1 的转录因子 GABARAPL1 的特异性蛋白 1 和 TATA 结合蛋白的核转位,通过降低其 O-GlcNAcNA 酰化水平来抑制它们的核转位,从而在再灌注期间反馈抑制星形胶质细胞中的 GABARAPL1。通过过度表达 GABARAPL1 恢复星形胶质细胞的糖吞噬功能可减少星形胶质细胞中的 DNA 损伤和氧化损伤,并提高再灌注期间周围神经元的存活率。此外,脑再灌注后急性期的低热量饮食可以增强星形胶质细胞的食糖通量,加速神经功能恢复。总之,大脑中的糖吞噬将自噬、代谢和表观遗传学联系在一起,糖吞噬功能障碍会加剧缺血性脑卒中后的再灌注损伤。
更新日期:2024-06-07
中文翻译:

星形细胞糖吞噬功能障碍加剧缺血性中风的再灌注损伤
糖吞噬已从一种替代的糖原降解途径演变为调节外周组织细胞代谢止血的多方面枢纽。然而,大脑中的糖吞噬模式及其对缺血性中风的潜在治疗作用仍然未知。在这里,我们观察到缺血性中风患者和小鼠再灌注期间星形胶质细胞糖吞噬功能障碍是由 GABA A 型受体相关蛋白样 1 (GABARAPL1) 的下调引起的。 PI3K-Akt 通路激活参与脑再灌注期间 GABARAPL1 下调。此外,糖吞噬功能障碍诱导的葡萄糖胺缺乏通过降低 GABARAPL1 转录因子 GABARAPL1 的转录因子 GABARAPL1 的特异性蛋白 1 和 TATA 结合蛋白的核转位,通过降低其 O-GlcNAcNA 酰化水平来抑制它们的核转位,从而在再灌注期间反馈抑制星形胶质细胞中的 GABARAPL1。通过过度表达 GABARAPL1 恢复星形胶质细胞的糖吞噬功能可减少星形胶质细胞中的 DNA 损伤和氧化损伤,并提高再灌注期间周围神经元的存活率。此外,脑再灌注后急性期的低热量饮食可以增强星形胶质细胞的食糖通量,加速神经功能恢复。总之,大脑中的糖吞噬将自噬、代谢和表观遗传学联系在一起,糖吞噬功能障碍会加剧缺血性脑卒中后的再灌注损伤。






































 京公网安备 11010802027423号
京公网安备 11010802027423号