当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cathepsin C from extracellular histone-induced M1 alveolar macrophages promotes NETosis during lung ischemia-reperfusion injury
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-06 , DOI: 10.1016/j.redox.2024.103231 Jing Yu , Yu Fu , Jiameng Gao , Qingqing Zhang , Nan Zhang , Zhiyuan Zhang , Xuemei Jiang , Chang Chen , Zongmei Wen
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-06 , DOI: 10.1016/j.redox.2024.103231 Jing Yu , Yu Fu , Jiameng Gao , Qingqing Zhang , Nan Zhang , Zhiyuan Zhang , Xuemei Jiang , Chang Chen , Zongmei Wen
|
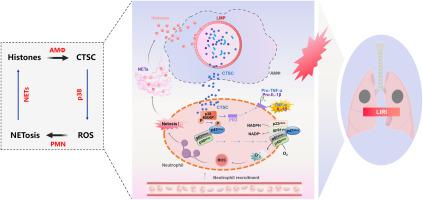
Primary graft dysfunction (PGD) is a severe form of acute lung injury resulting from lung ischemia/reperfusion injury (I/R) in lung transplantation (LTx), associated with elevated post-transplant morbidity and mortality rates. Neutrophils infiltrating during reperfusion are identified as pivotal contributors to lung I/R injury by releasing excessive neutrophil extracellular traps (NETs) via NETosis. While alveolar macrophages (AMs) are involved in regulating neutrophil chemotaxis and infiltration, their role in NETosis during lung I/R remains inadequately elucidated. Extracellular histones constitute the main structure of NETs and can activate AMs. In this study, we confirmed the significant involvement of extracellular histone-induced M1 phenotype of AMs (M1-AMs) in driving NETosis during lung I/R. Using secretome analysis, public protein databases, and transwell co-culture models of AMs and neutrophils, we identified Cathepsin C (CTSC) derived from AMs as a major mediator in NETosis. Further elucidating the molecular mechanisms, we found that CTSC induced NETosis through a pathway dependent on NADPH oxidase-mediated production of reactive oxygen species (ROS). CTSC could significantly activate p38 MAPK, resulting in the phosphorylation of the NADPH oxidase subunit p47, thereby facilitating the trafficking of cytoplasmic subunits to the cell membrane and activating NADPH oxidase. Moreover, CTSC up-regulated and activated its substrate membrane proteinase 3 (mPR3), resulting in an increased release of NETosis-related inflammatory factors. Inhibiting CTSC revealed great potential in mitigating NETosis-related injury during lung I/R. These findings suggests that CTSC from AMs may be a crucial factor in mediating NETosis during lung I/R, and targeting CTSC inhition may represent a novel intervention for PGD in LTx.
中文翻译:

细胞外组蛋白诱导的 M1 肺泡巨噬细胞组织蛋白酶 C 促进肺缺血再灌注损伤期间的 NETosis
原发性移植物功能障碍(PGD)是肺移植(LTx)中肺缺血/再灌注损伤(I/R)引起的一种严重的急性肺损伤,与移植后发病率和死亡率升高相关。再灌注期间浸润的中性粒细胞通过 NETosis 释放过量的中性粒细胞胞外陷阱 (NET),被认为是肺 I/R 损伤的关键因素。虽然肺泡巨噬细胞 (AM) 参与调节中性粒细胞趋化性和浸润,但它们在肺 I/R 期间 NETosis 中的作用仍未得到充分阐明。细胞外组蛋白构成NETs的主要结构,可以激活AMs。在这项研究中,我们证实了细胞外组蛋白诱导的 AMs (M1-AMs) M1 表型在肺 I/R 期间驱动 NETosis 中发挥着重要作用。利用分泌组分析、公共蛋白质数据库以及 AM 和中性粒细胞的 Transwell 共培养模型,我们确定源自 AM 的组织蛋白酶 C (CTSC) 是 NETosis 的主要介质。进一步阐明分子机制,我们发现 CTSC 通过依赖 NADPH 氧化酶介导的活性氧 (ROS) 产生的途径诱导 NETosis。 CTSC可显着激活p38 MAPK,导致NADPH氧化酶亚基p47磷酸化,从而促进细胞质亚基转运至细胞膜并激活NADPH氧化酶。此外,CTSC 上调并激活其底物膜蛋白酶 3 (mPR3),导致 NETosis 相关炎症因子的释放增加。抑制 CTSC 在减轻肺 I/R 期间 NETosis 相关损伤方面具有巨大潜力。 这些发现表明,来自 AM 的 CTSC 可能是介导肺 I/R 期间 NETosis 的关键因素,而针对 CTSC 抑制可能代表了 LTx 中 PGD 的一种新干预措施。
更新日期:2024-06-06
中文翻译:

细胞外组蛋白诱导的 M1 肺泡巨噬细胞组织蛋白酶 C 促进肺缺血再灌注损伤期间的 NETosis
原发性移植物功能障碍(PGD)是肺移植(LTx)中肺缺血/再灌注损伤(I/R)引起的一种严重的急性肺损伤,与移植后发病率和死亡率升高相关。再灌注期间浸润的中性粒细胞通过 NETosis 释放过量的中性粒细胞胞外陷阱 (NET),被认为是肺 I/R 损伤的关键因素。虽然肺泡巨噬细胞 (AM) 参与调节中性粒细胞趋化性和浸润,但它们在肺 I/R 期间 NETosis 中的作用仍未得到充分阐明。细胞外组蛋白构成NETs的主要结构,可以激活AMs。在这项研究中,我们证实了细胞外组蛋白诱导的 AMs (M1-AMs) M1 表型在肺 I/R 期间驱动 NETosis 中发挥着重要作用。利用分泌组分析、公共蛋白质数据库以及 AM 和中性粒细胞的 Transwell 共培养模型,我们确定源自 AM 的组织蛋白酶 C (CTSC) 是 NETosis 的主要介质。进一步阐明分子机制,我们发现 CTSC 通过依赖 NADPH 氧化酶介导的活性氧 (ROS) 产生的途径诱导 NETosis。 CTSC可显着激活p38 MAPK,导致NADPH氧化酶亚基p47磷酸化,从而促进细胞质亚基转运至细胞膜并激活NADPH氧化酶。此外,CTSC 上调并激活其底物膜蛋白酶 3 (mPR3),导致 NETosis 相关炎症因子的释放增加。抑制 CTSC 在减轻肺 I/R 期间 NETosis 相关损伤方面具有巨大潜力。 这些发现表明,来自 AM 的 CTSC 可能是介导肺 I/R 期间 NETosis 的关键因素,而针对 CTSC 抑制可能代表了 LTx 中 PGD 的一种新干预措施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号