当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering pathophysiological mechanisms underlying cystathionine beta-synthase-deficient homocystinuria using targeted metabolomics, liver proteomics, sphingolipidomics and analysis of mitochondrial function
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-04 , DOI: 10.1016/j.redox.2024.103222 Tomas Majtan 1 , Thomas Olsen 2 , Jitka Sokolova 3 , Jakub Krijt 3 , Michaela Křížková 3 , Tomoaki Ida 4 , Tamás Ditrói 5 , Hana Hansikova 3 , Ondrej Vit 6 , Jiri Petrak 6 , Ladislav Kuchař 3 , Warren D Kruger 7 , Péter Nagy 8 , Takaaki Akaike 4 , Viktor Kožich 9
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-04 , DOI: 10.1016/j.redox.2024.103222 Tomas Majtan 1 , Thomas Olsen 2 , Jitka Sokolova 3 , Jakub Krijt 3 , Michaela Křížková 3 , Tomoaki Ida 4 , Tamás Ditrói 5 , Hana Hansikova 3 , Ondrej Vit 6 , Jiri Petrak 6 , Ladislav Kuchař 3 , Warren D Kruger 7 , Péter Nagy 8 , Takaaki Akaike 4 , Viktor Kožich 9
Affiliation
|
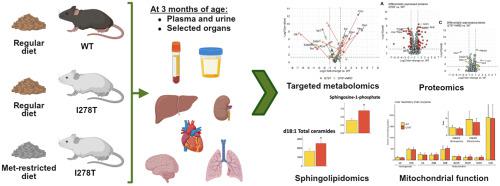
Cystathionine β-synthase (CBS)-deficient homocystinuria (HCU) is an inherited disorder of sulfur amino acid metabolism with varying severity and organ complications, and a limited knowledge about underlying pathophysiological processes. Here we aimed at getting an in-depth insight into disease mechanisms using a transgenic mouse model of HCU (I278T). We assessed metabolic, proteomic and sphingolipidomic changes, and mitochondrial function in tissues and body fluids of I278T mice and WT controls. Furthermore, we evaluated the efficacy of methionine-restricted diet (MRD) in I278T mice. In WT mice, we observed a distinct tissue/body fluid compartmentalization of metabolites with up to six-orders of magnitude differences in concentrations among various organs. The I278T mice exhibited the anticipated metabolic imbalance with signs of an increased production of hydrogen sulfide and disturbed persulfidation of free aminothiols. HCU resulted in a significant dysregulation of liver proteome affecting biological oxidations, conjugation of compounds, and metabolism of amino acids, vitamins, cofactors and lipids. Liver sphingolipidomics indicated upregulation of the pro-proliferative sphingosine-1-phosphate signaling pathway. Liver mitochondrial function of HCU mice did not seem to be impaired compared to controls. MRD in I278T mice improved metabolic balance in all tissues and substantially reduced dysregulation of liver proteome. The study highlights distinct tissue compartmentalization of sulfur-related metabolites in normal mice, extensive metabolome, proteome and sphingolipidome disruptions in I278T mice, and the efficacy of MRD to alleviate some of the HCU-related biochemical abnormalities.
中文翻译:

利用靶向代谢组学、肝脏蛋白质组学、鞘脂组学和线粒体功能分析破译胱硫醚β-合酶缺陷型高胱氨酸尿症的病理生理机制
胱硫醚β-合酶(CBS)缺陷型同型半胱氨酸尿症(HCU)是一种硫氨基酸代谢遗传性疾病,具有不同的严重程度和器官并发症,并且对潜在病理生理过程的了解有限。在这里,我们的目标是使用 HCU (I278T) 转基因小鼠模型深入了解疾病机制。我们评估了 I278T 小鼠和 WT 对照小鼠组织和体液中的代谢、蛋白质组和鞘脂组变化以及线粒体功能。此外,我们评估了 I278T 小鼠中蛋氨酸限制饮食 (MRD) 的功效。在 WT 小鼠中,我们观察到代谢物具有明显的组织/体液区室化,各个器官之间的浓度差异高达六个数量级。 I278T 小鼠表现出预期的代谢失衡,有硫化氢产生增加和游离氨基硫醇过硫化受到干扰的迹象。 HCU 导致肝脏蛋白质组显着失调,影响生物氧化、化合物结合以及氨基酸、维生素、辅因子和脂质的代谢。肝脏鞘脂组学表明促增殖鞘氨醇-1-磷酸信号通路上调。与对照组相比,HCU 小鼠的肝脏线粒体功能似乎没有受损。 I278T 小鼠的 MRD 改善了所有组织的代谢平衡,并显着减少了肝脏蛋白质组的失调。该研究强调了正常小鼠中硫相关代谢物的独特组织区划、I278T 小鼠中广泛的代谢组、蛋白质组和鞘脂组破坏,以及 MRD 缓解一些 HCU 相关生化异常的功效。
更新日期:2024-06-04
中文翻译:

利用靶向代谢组学、肝脏蛋白质组学、鞘脂组学和线粒体功能分析破译胱硫醚β-合酶缺陷型高胱氨酸尿症的病理生理机制
胱硫醚β-合酶(CBS)缺陷型同型半胱氨酸尿症(HCU)是一种硫氨基酸代谢遗传性疾病,具有不同的严重程度和器官并发症,并且对潜在病理生理过程的了解有限。在这里,我们的目标是使用 HCU (I278T) 转基因小鼠模型深入了解疾病机制。我们评估了 I278T 小鼠和 WT 对照小鼠组织和体液中的代谢、蛋白质组和鞘脂组变化以及线粒体功能。此外,我们评估了 I278T 小鼠中蛋氨酸限制饮食 (MRD) 的功效。在 WT 小鼠中,我们观察到代谢物具有明显的组织/体液区室化,各个器官之间的浓度差异高达六个数量级。 I278T 小鼠表现出预期的代谢失衡,有硫化氢产生增加和游离氨基硫醇过硫化受到干扰的迹象。 HCU 导致肝脏蛋白质组显着失调,影响生物氧化、化合物结合以及氨基酸、维生素、辅因子和脂质的代谢。肝脏鞘脂组学表明促增殖鞘氨醇-1-磷酸信号通路上调。与对照组相比,HCU 小鼠的肝脏线粒体功能似乎没有受损。 I278T 小鼠的 MRD 改善了所有组织的代谢平衡,并显着减少了肝脏蛋白质组的失调。该研究强调了正常小鼠中硫相关代谢物的独特组织区划、I278T 小鼠中广泛的代谢组、蛋白质组和鞘脂组破坏,以及 MRD 缓解一些 HCU 相关生化异常的功效。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号