当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of 3-(9H-carbazol-9-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoic acids as promising DNMT1 inhibitors
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-05-27 , DOI: 10.1016/j.ejmech.2024.116538 Jingyi Liu 1 , Minli Ruan 1 , Yueqin Liu 1 , Xiaoqian Hong 1 , Lijun Zhang 2 , Qian Zhang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-05-27 , DOI: 10.1016/j.ejmech.2024.116538 Jingyi Liu 1 , Minli Ruan 1 , Yueqin Liu 1 , Xiaoqian Hong 1 , Lijun Zhang 2 , Qian Zhang 1
Affiliation
|
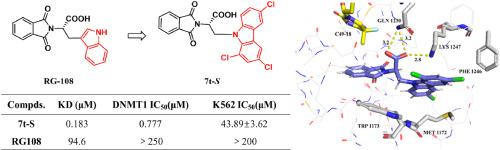
DNA methyltransferase 1 (DNMT1) is the primary enzyme responsible for maintaining DNA methylation patterns during cellular division, crucial for cancer development by suppressing tumor suppressor genes. In this study, we retained the phthalimide structure of -phthaloyl--tryptophan (RG108) and substituted its indole ring with nitrogen-containing aromatic rings of varying sizes. We synthesized 3-(9-carbazol-9-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoic acids and confirmed them as DNMT1 inhibitors through protein affinity testing, radiometric method using tritium labeled SAM, and MTT assay. Preliminary structure-activity relationship analysis revealed that introducing substituents on the carbazole ring could enhance inhibitory activity, with -configuration isomers showing greater activity than -configuration ones. Notably, -3-(3,6-di--butyl-9-carbazol-9-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoic acid () and -3-(1,3,6-trichloro-9-carbazol-9-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoic acid () exhibited significant DNMT1 enzyme inhibition activity, with IC values of 8.147 μM and 0.777 μM, respectively (compared to RG108 with an IC above 250 μM). Moreover, they demonstrated potential anti-proliferative activity on various tumor cell lines including A2780, HeLa, K562, and SiHa. Transcriptome analysis and KEGG pathway enrichment of K562 cells treated with and identified differentially expressed genes (DEGs) related to apoptosis and cell cycle pathways. Flow cytometry assays further indicated that and induce apoptosis in K562 cells and arrest them in the G0/G1 phase in a concentration-dependent manner. Molecular docking revealed that may bind to the methyl donor -adenosyl--methionine (SAM) site in DNMT1 with an orientation opposite to RG108, suggesting potential for deeper penetration into the DNMT1 pocket and laying the groundwork for further modifications.
中文翻译:

鉴定 3-(9H-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸作为有前途的 DNMT1 抑制剂
DNA 甲基转移酶 1 (DNMT1) 是负责在细胞分裂过程中维持 DNA 甲基化模式的主要酶,通过抑制抑癌基因对癌症的发展至关重要。在本研究中,我们保留了邻苯二甲酰色氨酸(RG108)的邻苯二甲酰亚胺结构,并用不同大小的含氮芳环取代了其吲哚环。我们合成了 3-(9-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸,并通过蛋白质亲和力测试、使用氚标记 SAM 的放射测量方法和 MTT 测定确认其为 DNMT1 抑制剂。初步构效关系分析表明,在咔唑环上引入取代基可以增强抑制活性,β构型异构体比β构型异构体表现出更大的活性。值得注意的是,-3-(3,6-二-丁基-9-咔唑-9-基)-2-(1,3-二氧代异二氢吲哚-2-基)丙酸()和-3-(1,3, 6-三氯-9-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸()表现出显着的 DNMT1 酶抑制活性,IC 值分别为 8.147 μM 和 0.777 μM(比较到 IC 高于 250 μM 的 RG108)。此外,它们还表现出对各种肿瘤细胞系(包括 A2780、HeLa、K562 和 SiHa)的潜在抗增殖活性。对经处理并鉴定出与细胞凋亡和细胞周期途径相关的差异表达基因 (DEG) 的 K562 细胞进行转录组分析和 KEGG 途径富集。流式细胞术分析进一步表明,并以浓度依赖性方式诱导 K562 细胞凋亡并使其停滞在 G0/G1 期。 分子对接显示,它可能以与 RG108 相反的方向与 DNMT1 中的甲基供体 - 腺苷 - 蛋氨酸 (SAM) 位点结合,表明有可能更深地渗透到 DNMT1 口袋中,并为进一步修饰奠定基础。
更新日期:2024-05-27
中文翻译:

鉴定 3-(9H-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸作为有前途的 DNMT1 抑制剂
DNA 甲基转移酶 1 (DNMT1) 是负责在细胞分裂过程中维持 DNA 甲基化模式的主要酶,通过抑制抑癌基因对癌症的发展至关重要。在本研究中,我们保留了邻苯二甲酰色氨酸(RG108)的邻苯二甲酰亚胺结构,并用不同大小的含氮芳环取代了其吲哚环。我们合成了 3-(9-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸,并通过蛋白质亲和力测试、使用氚标记 SAM 的放射测量方法和 MTT 测定确认其为 DNMT1 抑制剂。初步构效关系分析表明,在咔唑环上引入取代基可以增强抑制活性,β构型异构体比β构型异构体表现出更大的活性。值得注意的是,-3-(3,6-二-丁基-9-咔唑-9-基)-2-(1,3-二氧代异二氢吲哚-2-基)丙酸()和-3-(1,3, 6-三氯-9-咔唑-9-基)-2-(1,3-二氧异吲哚啉-2-基)丙酸()表现出显着的 DNMT1 酶抑制活性,IC 值分别为 8.147 μM 和 0.777 μM(比较到 IC 高于 250 μM 的 RG108)。此外,它们还表现出对各种肿瘤细胞系(包括 A2780、HeLa、K562 和 SiHa)的潜在抗增殖活性。对经处理并鉴定出与细胞凋亡和细胞周期途径相关的差异表达基因 (DEG) 的 K562 细胞进行转录组分析和 KEGG 途径富集。流式细胞术分析进一步表明,并以浓度依赖性方式诱导 K562 细胞凋亡并使其停滞在 G0/G1 期。 分子对接显示,它可能以与 RG108 相反的方向与 DNMT1 中的甲基供体 - 腺苷 - 蛋氨酸 (SAM) 位点结合,表明有可能更深地渗透到 DNMT1 口袋中,并为进一步修饰奠定基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号