Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NLRC5 senses NAD+ depletion, forming a PANoptosome and driving PANoptosis and inflammation
Cell ( IF 45.5 ) Pub Date : 2024-06-14 , DOI: 10.1016/j.cell.2024.05.034 Balamurugan Sundaram 1 , Nagakannan Pandian 1 , Hee Jin Kim 1 , Hadia M Abdelaal 1 , Raghvendra Mall 1 , Omkar Indari 1 , Roman Sarkar 1 , Rebecca E Tweedell 1 , Emily Q Alonzo 2 , Jonathon Klein 3 , Shondra M Pruett-Miller 3 , Peter Vogel 4 , Thirumala-Devi Kanneganti 1
Cell ( IF 45.5 ) Pub Date : 2024-06-14 , DOI: 10.1016/j.cell.2024.05.034 Balamurugan Sundaram 1 , Nagakannan Pandian 1 , Hee Jin Kim 1 , Hadia M Abdelaal 1 , Raghvendra Mall 1 , Omkar Indari 1 , Roman Sarkar 1 , Rebecca E Tweedell 1 , Emily Q Alonzo 2 , Jonathon Klein 3 , Shondra M Pruett-Miller 3 , Peter Vogel 4 , Thirumala-Devi Kanneganti 1
Affiliation
|
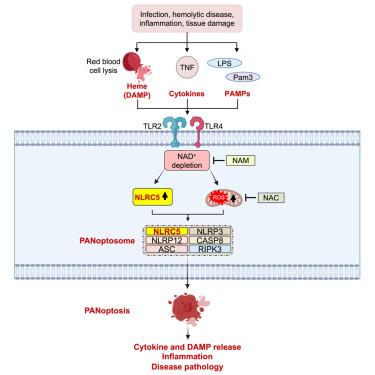
NLRs constitute a large, highly conserved family of cytosolic pattern recognition receptors that are central to health and disease, making them key therapeutic targets. NLRC5 is an enigmatic NLR with mutations associated with inflammatory and infectious diseases, but little is known about its function as an innate immune sensor and cell death regulator. Therefore, we screened for NLRC5’s role in response to infections, PAMPs, DAMPs, and cytokines. We identified that NLRC5 acts as an innate immune sensor to drive inflammatory cell death, PANoptosis, in response to specific ligands, including PAMP/heme and heme/cytokine combinations. NLRC5 interacted with NLRP12 and PANoptosome components to form a cell death complex, suggesting an NLR network forms similar to those in plants. Mechanistically, TLR signaling and NAD levels regulated NLRC5 expression and ROS production to control cell death. Furthermore, NLRC5-deficient mice were protected in hemolytic and inflammatory models, suggesting that NLRC5 could be a potential therapeutic target.
中文翻译:

NLRC5 感知 NAD+ 耗尽,形成 PANoptosome 并驱动 PANoptosis 和炎症
NLR 构成了一个大型、高度保守的胞质模式识别受体家族,对健康和疾病至关重要,使其成为关键的治疗靶点。 NLRC5 是一种神秘的 NLR,其突变与炎症和传染病相关,但人们对其作为先天免疫传感器和细胞死亡调节剂的功能知之甚少。因此,我们筛选了 NLRC5 在响应感染、PAMP、DAMP 和细胞因子中的作用。我们发现 NLRC5 作为一种先天免疫传感器,可响应特定配体(包括 PAMP/血红素和血红素/细胞因子组合)驱动炎症细胞死亡(PANoptosis)。 NLRC5 与 NLRP12 和 PANoptosome 成分相互作用形成细胞死亡复合物,表明 NLR 网络的形成类似于植物中的网络。从机制上讲,TLR 信号传导和 NAD 水平调节 NLRC5 表达和 ROS 产生以控制细胞死亡。此外,NLRC5 缺陷小鼠在溶血和炎症模型中受到保护,表明 NLRC5 可能是一个潜在的治疗靶点。
更新日期:2024-06-14
中文翻译:

NLRC5 感知 NAD+ 耗尽,形成 PANoptosome 并驱动 PANoptosis 和炎症
NLR 构成了一个大型、高度保守的胞质模式识别受体家族,对健康和疾病至关重要,使其成为关键的治疗靶点。 NLRC5 是一种神秘的 NLR,其突变与炎症和传染病相关,但人们对其作为先天免疫传感器和细胞死亡调节剂的功能知之甚少。因此,我们筛选了 NLRC5 在响应感染、PAMP、DAMP 和细胞因子中的作用。我们发现 NLRC5 作为一种先天免疫传感器,可响应特定配体(包括 PAMP/血红素和血红素/细胞因子组合)驱动炎症细胞死亡(PANoptosis)。 NLRC5 与 NLRP12 和 PANoptosome 成分相互作用形成细胞死亡复合物,表明 NLR 网络的形成类似于植物中的网络。从机制上讲,TLR 信号传导和 NAD 水平调节 NLRC5 表达和 ROS 产生以控制细胞死亡。此外,NLRC5 缺陷小鼠在溶血和炎症模型中受到保护,表明 NLRC5 可能是一个潜在的治疗靶点。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号