当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improving the genetic stability of bacterial growth control for long-term bioproduction
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-06-15 , DOI: 10.1002/bit.28756 Thibault Clavier 1, 2 , Corinne Pinel 1, 2 , Hidde de Jong 1, 2 , Johannes Geiselmann 1, 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-06-15 , DOI: 10.1002/bit.28756 Thibault Clavier 1, 2 , Corinne Pinel 1, 2 , Hidde de Jong 1, 2 , Johannes Geiselmann 1, 2
Affiliation
|
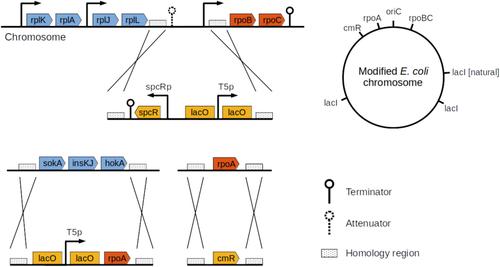
Using microorganisms for bioproduction requires the reorientation of metabolic fluxes from biomass synthesis to the production of compounds of interest. We previously engineered a synthetic growth switch in Escherichia coli based on inducible expression of the β- and β′-subunits of RNA polymerase. Depending on the level of induction, the cells stop growing or grow at a rate close to that of the wild-type strain. This strategy has been successful in transforming growth-arrested bacteria into biofactories with a high production yield, releasing cellular resources from growth towards biosynthesis. However, high selection pressure is placed on a growth-arrested population, favoring mutations that allow cells to escape from growth control. Accordingly, we made the design of the growth switch more robust by building in genetic redundancy. More specifically, we added the rpoA gene, encoding for the α-subunit of RNA polymerase, under the control of a copy of the same inducible promoter used for expression control of ββ′. The improved growth switch is much more stable (escape frequency <10−9), while preserving the capacity to improve production yields. Moreover, after a long period of growth inhibition the population can be regenerated within a few generations. This opens up the possibility to alternate biomass accumulation and product synthesis over a longer period of time and is an additional step towards the dynamical control of bioproduction.
中文翻译:

提高长期生物生产的细菌生长控制的遗传稳定性
使用微生物进行生物生产需要重新调整从生物质合成到目标化合物生产的代谢通量。我们之前基于 RNA 聚合酶 β 和 β' 亚基的诱导表达,在大肠杆菌中设计了一个合成生长开关。根据诱导水平,细胞停止生长或以接近野生型菌株的速度生长。该策略成功地将生长停滞的细菌转化为高产量的生物工厂,将细胞资源从生长释放到生物合成。然而,生长停滞的群体面临着高选择压力,有利于细胞逃避生长控制的突变。因此,我们通过构建遗传冗余使生长开关的设计更加稳健。更具体地说,我们添加了rpoA基因,该基因编码 RNA 聚合酶的 α 亚基,并受用于 ββ' 表达控制的相同诱导型启动子副本的控制。改进的生长开关更加稳定(逃逸频率 <10 id=2> − 9 ),同时保留提高产量的能力。此外,经过长期的生长抑制后,种群可以在几代之内再生。这开辟了在较长时间内交替生物质积累和产物合成的可能性,并且是朝着生物生产动态控制迈出的又一步。
更新日期:2024-06-15
中文翻译:

提高长期生物生产的细菌生长控制的遗传稳定性
使用微生物进行生物生产需要重新调整从生物质合成到目标化合物生产的代谢通量。我们之前基于 RNA 聚合酶 β 和 β' 亚基的诱导表达,在大肠杆菌中设计了一个合成生长开关。根据诱导水平,细胞停止生长或以接近野生型菌株的速度生长。该策略成功地将生长停滞的细菌转化为高产量的生物工厂,将细胞资源从生长释放到生物合成。然而,生长停滞的群体面临着高选择压力,有利于细胞逃避生长控制的突变。因此,我们通过构建遗传冗余使生长开关的设计更加稳健。更具体地说,我们添加了rpoA基因,该基因编码 RNA 聚合酶的 α 亚基,并受用于 ββ' 表达控制的相同诱导型启动子副本的控制。改进的生长开关更加稳定(逃逸频率 <10 id=2> − 9 ),同时保留提高产量的能力。此外,经过长期的生长抑制后,种群可以在几代之内再生。这开辟了在较长时间内交替生物质积累和产物合成的可能性,并且是朝着生物生产动态控制迈出的又一步。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号