当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Method to Large-Scalable Preparation of the Key Chiral Cyclohexane cis-Diamine Intermediate for Edoxaban
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-14 , DOI: 10.1021/acs.oprd.4c00039 Dingding Yuan 1 , Qiujun Peng 2 , Wenwen Xia 3 , Wansheng Yu 2 , Libo Ruan 2 , Yaping Wang 2 , Yu Chen 1 , Xianhua Pan 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-14 , DOI: 10.1021/acs.oprd.4c00039 Dingding Yuan 1 , Qiujun Peng 2 , Wenwen Xia 3 , Wansheng Yu 2 , Libo Ruan 2 , Yaping Wang 2 , Yu Chen 1 , Xianhua Pan 1
Affiliation
|
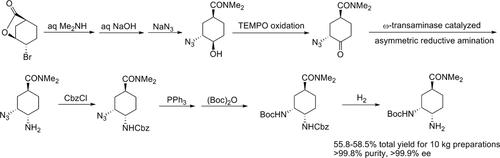
A new synthesis of tert-butyl (1R,2S,5S)-2-amino-5-(dimethylcarbamoyl)cyclohexylcarbamate, a key intermediate of edoxaban, is disclosed. This compound is a cyclohexane cis-diamine whose structure is a synthetic challenge. The known synthetic methods suffer from drawbacks, such as low yield, long reaction times, as well as excessive use of NaN3. The new method includes a total of nine steps starting from the known compound, (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one, whose γ-butyrolactone frame is ring-opened to form trans-3-azido-4-hydroxy cyclohexane after the first two steps. Subsequent oxidation leads to the formation of a cyclohexanone, which is then transformed to cis-diamine through enzyme-catalyzed asymmetric reductive amination, acylation with CbzCl, and reduction of the azido group successively. The target, tert-butyl (1R,2S,5S)-2-amino-5-(dimethylcarbamoyl)cyclohexylcarbamate, is finally formed through acylation with (Boc)2O, followed by deprotection of the Cbz group using hydrogenation.
中文翻译:

大规模制备艾多沙班关键手性环己烷顺二胺中间体的新方法
公开了艾多沙班的关键中间体(1R,2S,5S)-2-氨基-5-(二甲基氨基甲酰基)环己基氨基甲酸叔丁酯的新合成方法。该化合物是环己烷顺二胺,其结构是一个合成挑战。已知的合成方法存在诸如收率低、反应时间长以及NaN 3 的过度使用等缺点。新方法从已知化合物(1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one开始,总共包括九个步骤,其γ-丁内酯框架是开环的经过前两步后形成反式-3-叠氮基-4-羟基环己烷。随后的氧化导致环己酮的形成,然后通过酶催化的不对称还原胺化、CbzCl酰化和叠氮基的还原依次转化为顺式二胺。通过 (Boc) 2 O 酰化,然后脱除 Cbz 基团,最终形成目标物 (1R,2S,5S)-2-氨基-5-(二甲基氨基甲酰基)环己基氨基甲酸叔丁酯使用氢化。
更新日期:2024-06-14
中文翻译:

大规模制备艾多沙班关键手性环己烷顺二胺中间体的新方法
公开了艾多沙班的关键中间体(1R,2S,5S)-2-氨基-5-(二甲基氨基甲酰基)环己基氨基甲酸叔丁酯的新合成方法。该化合物是环己烷顺二胺,其结构是一个合成挑战。已知的合成方法存在诸如收率低、反应时间长以及NaN 3 的过度使用等缺点。新方法从已知化合物(1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one开始,总共包括九个步骤,其γ-丁内酯框架是开环的经过前两步后形成反式-3-叠氮基-4-羟基环己烷。随后的氧化导致环己酮的形成,然后通过酶催化的不对称还原胺化、CbzCl酰化和叠氮基的还原依次转化为顺式二胺。通过 (Boc) 2 O 酰化,然后脱除 Cbz 基团,最终形成目标物 (1R,2S,5S)-2-氨基-5-(二甲基氨基甲酰基)环己基氨基甲酸叔丁酯使用氢化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号