当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activating lattice oxygen by a defect-engineered Fe2O3–CeO2 nano-heterojunction for efficient electrochemical water oxidation
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-14 , DOI: 10.1039/d4ee01588f Qiuping Huang 1 , Guang-Jie Xia 2 , Bo Huang 1 , Dongling Xie 1 , Jianan Wang 1 , Dan Wen 1 , Dunmin Lin 1 , Chenggang Xu 1 , Lei Gao 3 , Zhenduo Wu 4 , Jinqi Wu 5 , Fengyu Xie 1 , Wenhan Guo 2 , Ruqiang Zou 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-14 , DOI: 10.1039/d4ee01588f Qiuping Huang 1 , Guang-Jie Xia 2 , Bo Huang 1 , Dongling Xie 1 , Jianan Wang 1 , Dan Wen 1 , Dunmin Lin 1 , Chenggang Xu 1 , Lei Gao 3 , Zhenduo Wu 4 , Jinqi Wu 5 , Fengyu Xie 1 , Wenhan Guo 2 , Ruqiang Zou 3
Affiliation
|
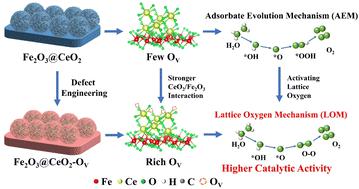
The sluggish anodic oxygen evolution reaction (OER) is currently the major hinderance for hydrogen production from water splitting. Iron-based materials are promising cost-effective candidates for OER electrocatalysts, however their low intrinsic activity limits their performance. Here we report a defect-engineered Fe2O3–CeO2 heterojunction with rich oxygen vacancies (Fe2O3@CeO2–OV), exhibiting ultralow overpotential of 172 mV at 10 mA cm−2 and 317 mV at 1000 mA cm−2, respectively, alongside superior stability and durability. Advanced characterization and density functional theory calculations demonstrate that defect engineering combined with heterojunction formation activates the lattice oxygen, switching the reaction pathway from the conventional adsorbate evolution mechanism (AEM) to the lattice oxygen mechanism (LOM). The oxygen vacancies are revealed to form preferably on CeO2 and induce not only electronic but also geometric modulation, contributing to strong Fe2O3–CeO2 interfacial interaction and charge transfer from CeO2 to Fe2O3, facilitating the O2 desorption and boosting the intrinsic activity.
中文翻译:

通过缺陷工程 Fe2O3-CeO2 纳米异质结激活晶格氧,实现高效电化学水氧化
缓慢的阳极析氧反应(OER)目前是水分解制氢的主要障碍。铁基材料是 OER 电催化剂的具有成本效益的候选材料,但其低固有活性限制了其性能。在这里,我们报告了一种缺陷设计的具有丰富氧空位的 Fe 2 O 3 –CeO 2 异质结(Fe 2 O 3 @CeO 2 –O V ),在 10 mA cm −2 下表现出 172 mV 的超低过电势,在 1000 mA cm −2 分别具有卓越的稳定性和耐用性。先进的表征和密度泛函理论计算表明,缺陷工程与异质结形成相结合可以激活晶格氧,将反应途径从传统的吸附质演化机制(AEM)切换到晶格氧机制(LOM)。研究表明,氧空位优选在 CeO 2 上形成,不仅会引起电子调制,还会引起几何调制,从而产生强 Fe 2 O 3 –CeO 2 从CeO 2 到Fe 2 O 3 的界面相互作用和电荷转移,促进O 2 解吸和升压内在活动。
更新日期:2024-06-14
中文翻译:

通过缺陷工程 Fe2O3-CeO2 纳米异质结激活晶格氧,实现高效电化学水氧化
缓慢的阳极析氧反应(OER)目前是水分解制氢的主要障碍。铁基材料是 OER 电催化剂的具有成本效益的候选材料,但其低固有活性限制了其性能。在这里,我们报告了一种缺陷设计的具有丰富氧空位的 Fe 2 O 3 –CeO 2 异质结(Fe 2 O 3 @CeO 2 –O V ),在 10 mA cm −2 下表现出 172 mV 的超低过电势,在 1000 mA cm −2 分别具有卓越的稳定性和耐用性。先进的表征和密度泛函理论计算表明,缺陷工程与异质结形成相结合可以激活晶格氧,将反应途径从传统的吸附质演化机制(AEM)切换到晶格氧机制(LOM)。研究表明,氧空位优选在 CeO 2 上形成,不仅会引起电子调制,还会引起几何调制,从而产生强 Fe 2 O 3 –CeO 2 从CeO 2 到Fe 2 O 3 的界面相互作用和电荷转移,促进O 2 解吸和升压内在活动。






































 京公网安备 11010802027423号
京公网安备 11010802027423号