当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
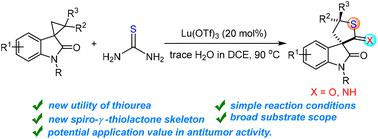
A domino [3 + 2] cycloaddition/deamination/imine hydrolysis reaction of cyclopropyl spirooxindoles with thiourea for access to spiro-γ-thiolactone oxindoles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-15 , DOI: 10.1039/d4qo00833b Hai-Ting Wang 1 , Xiao-Lin Wen 1 , Xiao-Lin Xu 1 , Dong-Chao Wang 1 , Hai-Ming Guo 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-15 , DOI: 10.1039/d4qo00833b Hai-Ting Wang 1 , Xiao-Lin Wen 1 , Xiao-Lin Xu 1 , Dong-Chao Wang 1 , Hai-Ming Guo 1
Affiliation
|
A domino [3 + 2] cycloaddition/deamination/imine hydrolysis reaction of D–A cyclopropane with inexpensive thiourea catalyzed by Lu(OTf)3 has been developed. A series of γ-thiolactone spirooxindole compounds with potential biological activity were obtained in up to 98% yields. Experiments to investigate the mechanism showed that γ-thiolactone products were from the hydrolysis of the imine intermediates. In addition, the asymmetric catalytic reaction of D–A cyclopropane and thiourea has been successfully developed to afford spiro-γ-thiolactone compounds with good enantioselectivities (up to 98% ee). The utility of this method was showcased by the facile transformation and biological activity of products.
中文翻译:

环丙基螺吲哚与硫脲的多米诺[3 + 2]环加成/脱氨/亚胺水解反应得到螺-γ-硫代内酯吲哚
开发了 Lu(OTf) 3 催化的 D-A 环丙烷与廉价硫脲的多米诺 [3 + 2] 环加成/脱氨/亚胺水解反应。获得了一系列具有潜在生物活性的γ-硫代内酯螺吲哚化合物,收率高达98%。机理实验表明,γ-硫代内酯产物来自于亚胺中间体的水解。此外,D-A环丙烷与硫脲的不对称催化反应已成功开发,可得到具有良好对映选择性(高达98% ee)的螺-γ-硫内酯化合物。该方法的实用性通过产物的简便转化和生物活性得到体现。
更新日期:2024-06-15
中文翻译:

环丙基螺吲哚与硫脲的多米诺[3 + 2]环加成/脱氨/亚胺水解反应得到螺-γ-硫代内酯吲哚
开发了 Lu(OTf) 3 催化的 D-A 环丙烷与廉价硫脲的多米诺 [3 + 2] 环加成/脱氨/亚胺水解反应。获得了一系列具有潜在生物活性的γ-硫代内酯螺吲哚化合物,收率高达98%。机理实验表明,γ-硫代内酯产物来自于亚胺中间体的水解。此外,D-A环丙烷与硫脲的不对称催化反应已成功开发,可得到具有良好对映选择性(高达98% ee)的螺-γ-硫内酯化合物。该方法的实用性通过产物的简便转化和生物活性得到体现。






































 京公网安备 11010802027423号
京公网安备 11010802027423号