Pharmaceutical Research ( IF 3.5 ) Pub Date : 2024-06-13 , DOI: 10.1007/s11095-024-03724-z Shanming Kuang 1 , Harsh S Shah 1 , Baoshu Zhao 2
|
Introduction
Hydrates are often used as pharmaceutical active pharmaceutical ingredients (API), especially when anhydrates may not be feasible likely due to physicochemical properties concerns. Pharmaceutical hydrates, whereas water is present as crystal adduct, are feasible for drug products as they do not pose any safety concern. Hydrates can impart many different advantages; therefore, they are quite common and preferred solid forms for numerous pharmaceutical materials on market. However, hydrates may involve various phase transitions, which may impact the stability and processability of drug substance.
Methods
Phase transitions, which include temperature-induced dehydration and moisture-facilitated rehydration are investigated by different solid-state analytical techniques such as powder x-ray diffraction, thermogravimetric analysis, differential scanning calorimetry, polarized light microscopy, and single-crystal x-ray diffraction.
Results
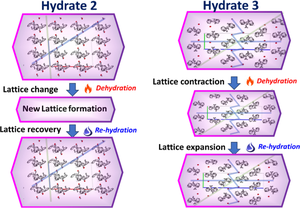
This research investigation focuses on the different phase transition behaviors of a newly discovered pharmaceutical compound with three channel hydrates, two of which confirmed by single-crystal analysis. The retention or rearrangement of crystal structures over the transitions are studied. Hydrate 3 exhibits a characteristic feature of channel hydrate that involves symmetric lattice relaxation. Unlike hydrate 3, hydrate 2 results in a potentially new unit cell upon dehydration due to asymmetric lattice relaxation, which converted back to Hydrate 2 in presence of water, a very unique behavior for a channel hydrate, rarely observed, which entails novelty of this research work.
Conclusion
The relationship among crystal forms of different hydrates of this new compound is thus established. The current investigation is a vital part of drug product risk assessment for hydrates to avoid any challenges during manufacturing operations and/or stability studies. This investigation was successfully applied in the present study and can be expanded to other newly discovered APIs in future.
Graphical Abstract
中文翻译:

涉及新药物化合物通道水合物的相变
介绍
水合物通常用作药物活性药物成分 (API),特别是当无水物可能由于物理化学性质问题而不可行时。药物水合物,而水以结晶加合物的形式存在,对于药品来说是可行的,因为它们不会造成任何安全问题。水合物可以赋予许多不同的优势;因此,它们是市场上许多制药材料非常常见和首选的固体形式。然而,水合物可能涉及各种相变,这可能会影响原料药的稳定性和可加工性。
方法
通过不同的固态分析技术(如粉末 X 射线衍射、热重分析、差示扫描量热法、偏振光显微镜和单晶 X 射线衍射)研究了相变,包括温度诱导脱水和水分促进再水化。
结果
本研究调查的重点是新发现的具有三个通道水合物的药物化合物的不同相变行为,其中两个通过单晶分析得到证实。研究了晶体结构在跃迁上的保留或重排。Hydrate 3 表现出通道 Hydrate 的特征,涉及对称晶格弛豫。与水合物 3 不同,水合物 2 由于不对称晶格弛豫而脱水时会产生潜在的新晶胞,在水存在下转化回水合物 2,这是通道水合物的一种非常独特的行为,很少观察到,这需要这项研究工作的新颖性。
结论
这样就建立了这种新化合物的不同水合物的晶型之间的关系。目前的调查是水合物药品风险评估的重要组成部分,以避免在生产操作和/或稳定性研究过程中出现任何挑战。这项研究已成功应用于本研究,将来可以扩展到其他新发现的 API。































 京公网安备 11010802027423号
京公网安备 11010802027423号