当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3·OEt2 Catalyzed Cascade [4 + 2] Benzannulation of Vinyloxiranes with Coumarins to Construct Benzocoumarin Derivatives
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-13 , DOI: 10.1021/acs.joc.4c00742 Yafei Wang 1 , Yujia Wang 1 , Jiaxin Qu 1 , Tongtong Yang 1 , Yining Zhang 1 , Chunhao Yuan 2 , Hongchao Guo 3 , Chang Wang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-13 , DOI: 10.1021/acs.joc.4c00742 Yafei Wang 1 , Yujia Wang 1 , Jiaxin Qu 1 , Tongtong Yang 1 , Yining Zhang 1 , Chunhao Yuan 2 , Hongchao Guo 3 , Chang Wang 1
Affiliation
|
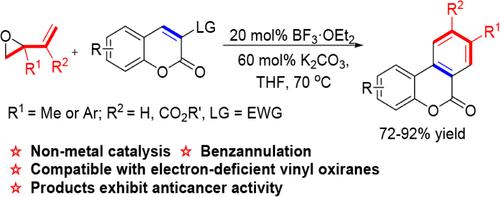
A BF3·OEt2-catalyzed cascade cyclization reaction of vinyloxirane with coumarin is described, affording the benzocoumarin derivatives with moderate to excellent yields (72–92%). The reaction demonstrates exceptional substrate tolerance and has been extensively explored for its potential in drug development, including scale-up experiments, functional group transformations, and screening of the products for anticancer activity. Moreover, the reaction mechanism has been rigorously validated through intermediate trapping and control experiments. Additionally, this reaction represents the uncommon nonmetal catalyzed intermolecular cyclization of vinyloxiranes.
中文翻译:

BF3·OEt2 催化级联[4 + 2]乙烯基环氧乙烷与香豆素苯并环化构建苯并香豆素衍生物
描述了 BF 3 ·OEt 2催化的乙烯基环氧乙烷与香豆素的级联环化反应,以中等至优异的收率 (72–92%) 提供苯并香豆素衍生物。该反应表现出卓越的底物耐受性,并因其在药物开发中的潜力而得到广泛探索,包括放大实验、官能团转化和产品抗癌活性筛选。此外,反应机制已通过中间捕获和控制实验得到严格验证。此外,该反应代表了不常见的非金属催化的乙烯基环氧乙烷分子间环化。
更新日期:2024-06-13
中文翻译:

BF3·OEt2 催化级联[4 + 2]乙烯基环氧乙烷与香豆素苯并环化构建苯并香豆素衍生物
描述了 BF 3 ·OEt 2催化的乙烯基环氧乙烷与香豆素的级联环化反应,以中等至优异的收率 (72–92%) 提供苯并香豆素衍生物。该反应表现出卓越的底物耐受性,并因其在药物开发中的潜力而得到广泛探索,包括放大实验、官能团转化和产品抗癌活性筛选。此外,反应机制已通过中间捕获和控制实验得到严格验证。此外,该反应代表了不常见的非金属催化的乙烯基环氧乙烷分子间环化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号