当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of Sulfilimines by Iron-Catalyzed Iminations of Sulfides with 2,2,2-Trichloroethyl Sulfamate
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-13 , DOI: 10.1021/acs.joc.4c01250 Kiruthika Periasamy 1 , Sofya Gordeeva 1 , Carsten Bolm 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-13 , DOI: 10.1021/acs.joc.4c01250 Kiruthika Periasamy 1 , Sofya Gordeeva 1 , Carsten Bolm 1
Affiliation
|
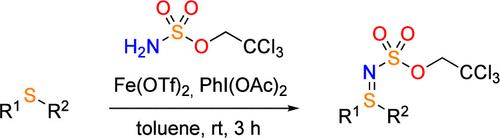
N-protected sulfilimines are prepared by imination of sulfides with a combination of 2,2,2-trichloroethyl sulfamate (H2NTces), (diacetoxyiodo)benzene (PIDA), and a catalytic amount of iron triflate. The reaction proceeds at room temperature, and after only 3 h a wide range of acyclic and cyclic NTces-sulfilimines with various functional groups and (hetero)aryl substituents can be obtained. By subsequent oxidation followed by deprotection, the products are converted into NH-sulfoximines.
中文翻译:

铁催化硫化物与 2,2,2-三氯氨基磺酸乙酯亚胺化合成硫亚胺
N-保护的硫亚胺通过硫化物与2,2,2-三氯乙基氨基磺酸盐(H 2 NTces)、(二乙酰氧基碘)苯(PIDA)和催化量的三氟甲磺酸铁的组合进行亚胺化来制备。该反应在室温下进行,仅3小时后即可获得具有各种官能团和(杂)芳基取代基的各种无环和环状N Tces-硫亚胺。通过随后的氧化和脱保护,产物转化为N H-亚磺酰亚胺。
更新日期:2024-06-13
中文翻译:

铁催化硫化物与 2,2,2-三氯氨基磺酸乙酯亚胺化合成硫亚胺
N-保护的硫亚胺通过硫化物与2,2,2-三氯乙基氨基磺酸盐(H 2 NTces)、(二乙酰氧基碘)苯(PIDA)和催化量的三氟甲磺酸铁的组合进行亚胺化来制备。该反应在室温下进行,仅3小时后即可获得具有各种官能团和(杂)芳基取代基的各种无环和环状N Tces-硫亚胺。通过随后的氧化和脱保护,产物转化为N H-亚磺酰亚胺。















































 京公网安备 11010802027423号
京公网安备 11010802027423号