当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nitrite in Pharmaceutical Manufacturing Water: Development of an Ultra-Sensitive Analytical Method, Typical Data, and Discussion of Potential Nitrosamine Formation in Drug Substance and Drug Product from Water
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-12 , DOI: 10.1021/acs.oprd.4c00037 A. B. Suresh Kumar 1 , Debasis Dey 1 , T. S. Balaji 1 , Haridoss Karthik 1 , K. Sathishkumar 1 , Paul McDaid 2 , Brian Fitzpatrick 3 , Atul Awasthi 1 , Simon Davies 2 , Olivier Dirat 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-12 , DOI: 10.1021/acs.oprd.4c00037 A. B. Suresh Kumar 1 , Debasis Dey 1 , T. S. Balaji 1 , Haridoss Karthik 1 , K. Sathishkumar 1 , Paul McDaid 2 , Brian Fitzpatrick 3 , Atul Awasthi 1 , Simon Davies 2 , Olivier Dirat 4
Affiliation
|
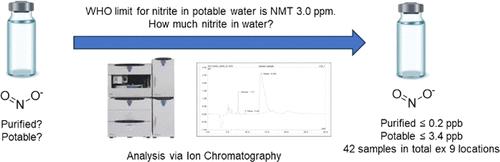
Recent observations of N-nitrosamine presence in nonsartan medicinal products have led to product recalls and regulatory requests for marketing authorization holders to assess their portfolios for chemically synthesized active pharmaceutical ingredients (APIs), associated drug products (DPs) and packaging for the potential presence of N-nitrosamines. As part of the overall risk assessment for nitrosamine formation, the identification of nitrosating agents potentially present during API or DP manufacture is required. Process water is ubiquitous to manufacturing, and consequently the accurate determination of nitrite content in process water will support an understanding of the potential for nitrosamine formation from nitrite in process water for a given API or DP manufacturing process. Herein, we report the development of two ultrasensitive ion chromatography analytical methods for the quantification of the amount of nitrite in purified and potable water, respectively. Various samples, including a range of water types and geographical locations, have been tested using these methods. Water samples from various sources from multiple manufacturing sites were successfully analyzed with the aid of these methods. Twenty-one out of twenty-two purified water samples analyzed had nitrite levels that were below the quantitation limit of the method, i.e. <0.1 ppb. All potable water samples analyzed had nitrite levels that were <3.5 ppb. The results are contextualized to illustrate the amounts of nitrosamine that could be formed from process water during API and DP manufacture. For API manufacturing, the use of purified water, even under acidic conditions and in the presence of vulnerable amines, will not lead to significant nitrosamine formation in the product because there is so little nitrite present. For potable water, in most cases, only operations performed under acidic conditions (pH less than 7) may lead to significant conversion of nitrite. Basic amines, typically dialkyl amines, with a pKa of 10 and more, will have negligible formation of their corresponding nitrosamines over a 24 h time period, even at the worst case pH of 3.15. Less basic amines (pKa of less than 10) under acidic conditions (pH less than 7) may lead to significant conversions within 24 h, and for these cases the overall nitrosamine formation risk will depend on the water type, the amount of water used, and the downstream purge opportunities in the manufacturing process. For drug products, the use of WFI generated by distillation poses no nitrosamine risk formation due to the low levels of nitrite. Likewise, purified water used in wet granulation drug product processes is a vanishingly small risk for nitrosamine formation. It is also an insignificant risk for nitrosamine formation in drug product formulation.
中文翻译:

制药用水中的亚硝酸盐:超灵敏分析方法的开发、典型数据以及水中原料药和药品中潜在亚硝胺形成的讨论
最近观察到非沙坦类药品中存在 N-亚硝胺,已导致产品召回,并要求上市许可持有者评估其化学合成活性药物成分 (API)、相关药品 (DP) 和包装组合中是否存在潜在的 N-亚硝胺。 N-亚硝胺。作为亚硝胺形成总体风险评估的一部分,需要识别 API 或 DP 制造过程中可能存在的亚硝化剂。工艺用水在生产过程中无处不在,因此,准确测定工艺用水中的亚硝酸盐含量将有助于了解给定 API 或 DP 生产工艺中工艺用水中的亚硝酸盐形成亚硝胺的可能性。在此,我们报告了两种超灵敏离子色谱分析方法的开发,分别用于定量纯净水和饮用水中的亚硝酸盐含量。各种样本,包括一系列水类型和地理位置,都已使用这些方法进行了测试。借助这些方法,成功地分析了来自多个生产基地的不同来源的水样。所分析的 22 个纯化水样品中有 21 个的亚硝酸盐含量低于该方法的定量限,即 <0.1 ppb。分析的所有饮用水样品的亚硝酸盐含量均<3.5 ppb。将结果结合起来说明 API 和 DP 制造过程中工艺用水可能形成的亚硝胺含量。 对于 API 生产,使用纯化水,即使在酸性条件下且存在易损胺的情况下,也不会导致产品中形成大量亚硝胺,因为亚硝酸盐含量极少。对于饮用水,在大多数情况下,只有在酸性条件(pH 值小于 7)下进行的操作才可能导致亚硝酸盐的显着转化。 pK a 为 10 或更高的碱性胺(通常为二烷基胺)在 24 小时内生成的相应亚硝胺的量可以忽略不计,即使在 pH 值为 3.15 的最坏情况下也是如此。酸性条件(pH 小于 7)下碱性胺较少(pK a 小于 10)可能会在 24 小时内导致显着转化,对于这些情况,总体亚硝胺形成风险将取决于水类型、用水量以及制造过程中的下游净化机会。对于药品,由于亚硝酸盐含量低,使用蒸馏产生的注射用水不会产生亚硝胺风险。同样,湿法制粒药品工艺中使用的纯化水形成亚硝胺的风险微乎其微。在药品制剂中形成亚硝胺的风险也很小。
更新日期:2024-06-13
中文翻译:

制药用水中的亚硝酸盐:超灵敏分析方法的开发、典型数据以及水中原料药和药品中潜在亚硝胺形成的讨论
最近观察到非沙坦类药品中存在 N-亚硝胺,已导致产品召回,并要求上市许可持有者评估其化学合成活性药物成分 (API)、相关药品 (DP) 和包装组合中是否存在潜在的 N-亚硝胺。 N-亚硝胺。作为亚硝胺形成总体风险评估的一部分,需要识别 API 或 DP 制造过程中可能存在的亚硝化剂。工艺用水在生产过程中无处不在,因此,准确测定工艺用水中的亚硝酸盐含量将有助于了解给定 API 或 DP 生产工艺中工艺用水中的亚硝酸盐形成亚硝胺的可能性。在此,我们报告了两种超灵敏离子色谱分析方法的开发,分别用于定量纯净水和饮用水中的亚硝酸盐含量。各种样本,包括一系列水类型和地理位置,都已使用这些方法进行了测试。借助这些方法,成功地分析了来自多个生产基地的不同来源的水样。所分析的 22 个纯化水样品中有 21 个的亚硝酸盐含量低于该方法的定量限,即 <0.1 ppb。分析的所有饮用水样品的亚硝酸盐含量均<3.5 ppb。将结果结合起来说明 API 和 DP 制造过程中工艺用水可能形成的亚硝胺含量。 对于 API 生产,使用纯化水,即使在酸性条件下且存在易损胺的情况下,也不会导致产品中形成大量亚硝胺,因为亚硝酸盐含量极少。对于饮用水,在大多数情况下,只有在酸性条件(pH 值小于 7)下进行的操作才可能导致亚硝酸盐的显着转化。 pK a 为 10 或更高的碱性胺(通常为二烷基胺)在 24 小时内生成的相应亚硝胺的量可以忽略不计,即使在 pH 值为 3.15 的最坏情况下也是如此。酸性条件(pH 小于 7)下碱性胺较少(pK a 小于 10)可能会在 24 小时内导致显着转化,对于这些情况,总体亚硝胺形成风险将取决于水类型、用水量以及制造过程中的下游净化机会。对于药品,由于亚硝酸盐含量低,使用蒸馏产生的注射用水不会产生亚硝胺风险。同样,湿法制粒药品工艺中使用的纯化水形成亚硝胺的风险微乎其微。在药品制剂中形成亚硝胺的风险也很小。






































 京公网安备 11010802027423号
京公网安备 11010802027423号