Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ultrasensitive quantification of PD-L1+ extracellular vesicles in melanoma patient plasma using a parallelized high throughput droplet digital assay
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-06-13 , DOI: 10.1039/d4lc00331d Hanfei Shen 1 , Yasemin Atiyas 1 , Zijian Yang 2 , Andrew A. Lin 1 , Jingbo Yang 3 , Diao Liu 3 , Juhwan Park 1 , Wei Guo 3 , David A. Issadore 1, 4
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-06-13 , DOI: 10.1039/d4lc00331d Hanfei Shen 1 , Yasemin Atiyas 1 , Zijian Yang 2 , Andrew A. Lin 1 , Jingbo Yang 3 , Diao Liu 3 , Juhwan Park 1 , Wei Guo 3 , David A. Issadore 1, 4
Affiliation
|
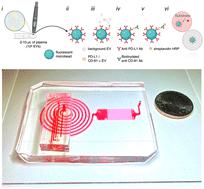
The expression of programmed death-ligand 1 (PD-L1) on extracellular vesicles (EVs) is an emerging biomarker for cancer, and has gained particular interest for its role mediating immunotherapy. However, precise quantification of PD-L1+ EVs in clinical samples remains challenging due to their sparse concentration and the enormity of the number of background EVs in human plasma, limiting applicability of conventional approaches. In this study, we develop a high-throughput droplet-based extracellular vesicle analysis (DEVA) assay for ultrasensitive quantification of EVs in plasma that are dual positive for both PD-L1 and tetraspanin (CD81) known to be expressed on EVs. We achieve a performance that significantly surpasses conventional approaches, demonstrating 360× enhancement in the limit of detection (LOD) and a 750× improvement in the limit of quantitation (LOQ) compared to conventional plate enzyme-linked immunoassay (ELISA). Underlying this performance is DEVA's high throughput analysis of individual EVs one at a time and the high specificity to targeted EVs versus background. We achieve a 0.006% false positive rate per droplet by leveraging avidity effects that arise from EVs having multiple copies of their target ligands on their surface. We use parallelized optofluidics to rapidly process 10 million droplets per minute, ∼100× greater than conventional approaches. A validation study on a cohort of 14 patients with melanoma confirms DEVA's ability to match conventional ELISA measurements with reduced plasma sample volume and without the need for prior EV purification. This proof-of-concept study demonstrates DEVA's potential for clinical utility to enhance prognosis as well as guide treatment for cancer.
中文翻译:

使用并行高通量液滴数字测定法对黑色素瘤患者血浆中的 PD-L1+ 细胞外囊泡进行超灵敏定量
细胞外囊泡 (EV) 上程序性死亡配体 1 (PD-L1) 的表达是一种新兴的癌症生物标志物,因其介导免疫治疗的作用而受到特别关注。然而,由于临床样本中 PD-L1+ EV 的浓度稀疏且人血浆中背景 EV 数量巨大,因此精确定量临床样本中的 PD-L1+ EV 仍然具有挑战性,限制了传统方法的适用性。在这项研究中,我们开发了一种基于高通量液滴的细胞外囊泡分析 (DEVA) 测定法,用于对血浆中的 EV 进行超灵敏定量,这些 EV 对已知在 EV 上表达的 PD-L1 和四跨膜蛋白 (CD81) 呈双阳性。我们取得了显着超越传统方法的性能,与传统板酶联免疫分析 (ELISA) 相比,检测限 (LOD) 提高了 360 倍,定量限 (LOQ) 提高了 750 倍。这一性能的基础是 DEVA 对单个 EV 的高通量分析,以及对目标 EV 与背景的高度特异性。通过利用表面具有多个目标配体副本的 EV 产生的亲合力效应,我们实现了每个液滴 0.006% 的假阳性率。我们使用并行光流控技术每分钟快速处理 1000 万个液滴,比传统方法高约 100 倍。对 14 名黑色素瘤患者进行的验证研究证实,DEVA 能够将传统 ELISA 测量与减少的血浆样本量相匹配,且无需事先进行 EV 纯化。这项概念验证研究证明了 DEVA 在增强预后以及指导癌症治疗方面具有临床实用性的潜力。
更新日期:2024-06-13
中文翻译:

使用并行高通量液滴数字测定法对黑色素瘤患者血浆中的 PD-L1+ 细胞外囊泡进行超灵敏定量
细胞外囊泡 (EV) 上程序性死亡配体 1 (PD-L1) 的表达是一种新兴的癌症生物标志物,因其介导免疫治疗的作用而受到特别关注。然而,由于临床样本中 PD-L1+ EV 的浓度稀疏且人血浆中背景 EV 数量巨大,因此精确定量临床样本中的 PD-L1+ EV 仍然具有挑战性,限制了传统方法的适用性。在这项研究中,我们开发了一种基于高通量液滴的细胞外囊泡分析 (DEVA) 测定法,用于对血浆中的 EV 进行超灵敏定量,这些 EV 对已知在 EV 上表达的 PD-L1 和四跨膜蛋白 (CD81) 呈双阳性。我们取得了显着超越传统方法的性能,与传统板酶联免疫分析 (ELISA) 相比,检测限 (LOD) 提高了 360 倍,定量限 (LOQ) 提高了 750 倍。这一性能的基础是 DEVA 对单个 EV 的高通量分析,以及对目标 EV 与背景的高度特异性。通过利用表面具有多个目标配体副本的 EV 产生的亲合力效应,我们实现了每个液滴 0.006% 的假阳性率。我们使用并行光流控技术每分钟快速处理 1000 万个液滴,比传统方法高约 100 倍。对 14 名黑色素瘤患者进行的验证研究证实,DEVA 能够将传统 ELISA 测量与减少的血浆样本量相匹配,且无需事先进行 EV 纯化。这项概念验证研究证明了 DEVA 在增强预后以及指导癌症治疗方面具有临床实用性的潜力。












































 京公网安备 11010802027423号
京公网安备 11010802027423号