当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free reaction of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates: synthesis of 1,2-dihydroquinolines and 2,3-dihydropyrroles
Chemical Communications ( IF 4.3 ) Pub Date : 2024-06-13 , DOI: 10.1039/d4cc02156h Tao Wang 1 , Zhongyue Lu 1 , Pengfei Li 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-06-13 , DOI: 10.1039/d4cc02156h Tao Wang 1 , Zhongyue Lu 1 , Pengfei Li 1
Affiliation
|
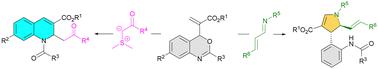
Catalyst-free annulations of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates have been successfully achieved under mild conditions. Specifically, the reaction of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates with sulfur ylides furnished various 1,2-dihydroquinolines in generally high yields. Furthermore, [3+2]-annulations of 2-(4H-benzo[d][1,3]oxazin-4-yl)acrylates with α,β-unsaturated imines afforded a broad scope of polysubstituted 2,3-dihydropyrroles with high efficiency.
中文翻译:

2-(4H-苯并[d][1,3]恶嗪-4-基)丙烯酸酯的无催化剂反应:合成1,2-二氢喹啉和2,3-二氢吡咯
在温和条件下成功实现了 2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯的无催化剂成环。具体而言,2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯与硫叶立德的反应通常以高收率提供各种1,2-二氢喹啉。此外,2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯与α,β-不饱和亚胺的[3+2]-环化提供了广泛的多取代2,3-二氢吡咯效率高。
更新日期:2024-06-18
中文翻译:

2-(4H-苯并[d][1,3]恶嗪-4-基)丙烯酸酯的无催化剂反应:合成1,2-二氢喹啉和2,3-二氢吡咯
在温和条件下成功实现了 2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯的无催化剂成环。具体而言,2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯与硫叶立德的反应通常以高收率提供各种1,2-二氢喹啉。此外,2-(4 H-苯并[ d ][1,3]恶嗪-4-基)丙烯酸酯与α,β-不饱和亚胺的[3+2]-环化提供了广泛的多取代2,3-二氢吡咯效率高。






























 京公网安备 11010802027423号
京公网安备 11010802027423号