当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical aminotrideuteromethylthiolation of isocyanides with anilines and CD3SSO3Na
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-12 , DOI: 10.1039/d4qo00858h Lin Zhao 1 , Xinyu Zhou 1 , Kemeng Zhang 1 , Siyu Han 1 , Ge Wu 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-12 , DOI: 10.1039/d4qo00858h Lin Zhao 1 , Xinyu Zhou 1 , Kemeng Zhang 1 , Siyu Han 1 , Ge Wu 1, 2
Affiliation
|
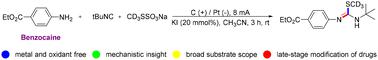
Herein, we describe an electrochemical strategy that enables the aminotrideuteromethylthiolation of isocyanides with anilines and CD3SSO3Na, providing an unprecedented route to access S-CD3 isothioureas in satisfactory yields. Mechanistic studies reveal that the transformation is initiated via the addition of the CD3S radical to the isocyanide, followed by a nucleophilic attack on the in situ generated imine carbocation intermediate by anilines. Importantly, these reactions exhibit exclusive chemoselectivity at the carbon of isocyanides and wide functional group compatibility as well as enable late-stage functionalization of complex substrates.
中文翻译:

异氰化物与苯胺和 CD3SSO3Na 的电化学氨基三氘代甲硫基化
在此,我们描述了一种电化学策略,该策略能够使异氰化物与苯胺和 CD 3 SSO 3 Na 发生氨基三氘代甲硫基化,从而提供前所未有的获取 S-CD 3 的途径异硫脲的收率令人满意。机理研究表明,该转化是通过将 CD 3 S 自由基添加到异氰化物中引发的,然后通过苯胺对原位生成的亚胺碳阳离子中间体进行亲核攻击。重要的是,这些反应对异氰化物的碳表现出独特的化学选择性和广泛的官能团相容性,并且能够实现复杂底物的后期功能化。
更新日期:2024-06-12
中文翻译:

异氰化物与苯胺和 CD3SSO3Na 的电化学氨基三氘代甲硫基化
在此,我们描述了一种电化学策略,该策略能够使异氰化物与苯胺和 CD 3 SSO 3 Na 发生氨基三氘代甲硫基化,从而提供前所未有的获取 S-CD 3 的途径异硫脲的收率令人满意。机理研究表明,该转化是通过将 CD 3 S 自由基添加到异氰化物中引发的,然后通过苯胺对原位生成的亚胺碳阳离子中间体进行亲核攻击。重要的是,这些反应对异氰化物的碳表现出独特的化学选择性和广泛的官能团相容性,并且能够实现复杂底物的后期功能化。






































 京公网安备 11010802027423号
京公网安备 11010802027423号