Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of heterochromatin remodeling revealed by the DDM1 bound nucleosome structures
Structure ( IF 4.4 ) Pub Date : 2024-06-12 , DOI: 10.1016/j.str.2024.05.013 Hongwei Zhang 1 , Zhanxi Gu 2 , Yuan Zeng 1 , Yu Zhang 1
Structure ( IF 4.4 ) Pub Date : 2024-06-12 , DOI: 10.1016/j.str.2024.05.013 Hongwei Zhang 1 , Zhanxi Gu 2 , Yuan Zeng 1 , Yu Zhang 1
Affiliation
|
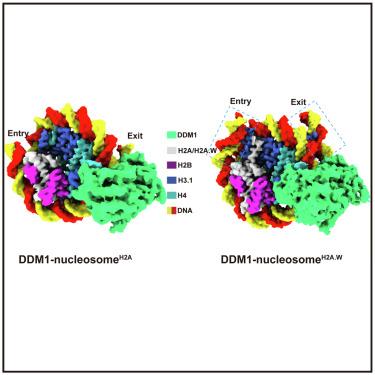
The SWI/SNF2 chromatin remodeling factor decreased DNA methylation 1 (DDM1) is essential for the silencing of transposable elements (TEs) in both euchromatic and heterochromatic regions. Here, we determined the cryo-EM structures of DDM1-nucleosome and DDM1-nucleosome complexes at near-atomic resolution in the presence of the ATP analog ADP-BeFx. The structures show that nucleosomal DNA is unwrapped more on the surface of the histone octamer containing histone H2A than that containing histone H2A.W. DDM1 embraces one DNA gyre of the nucleosome and interacts with the N-terminal tails of histone H4. Although we did not observe DDM1-H2A.W interactions in our structures, the results of the pull-down experiments suggest a direct interaction between DDM1 and the core region of histone H2A.W. Our work provides mechanistic insights into the heterochromatin remodeling process driven by DDM1 in plants.
中文翻译:

DDM1结合核小体结构揭示异染色质重塑机制
SWI/SNF2 染色质重塑因子降低 DNA 甲基化 1 (DDM1) 对于常染色质和异染色质区域转座元件 (TE) 的沉默至关重要。在这里,我们在 ATP 类似物 ADP-BeFx 存在的情况下以近原子分辨率测定了 DDM1-核小体和 DDM1-核小体复合物的冷冻电镜结构。该结构表明,与含有组蛋白 H2A.W 的组蛋白八聚体相比,核小体 DNA 在含有组蛋白 H2A 的组蛋白八聚体的表面上更多地展开。 DDM1 包含核小体的一个 DNA 环,并与组蛋白 H4 的 N 末端尾部相互作用。尽管我们没有在我们的结构中观察到 DDM1-H2A.W 相互作用,但下拉实验的结果表明 DDM1 和组蛋白 H2A.W 核心区域之间存在直接相互作用。我们的工作为植物中由 DDM1 驱动的异染色质重塑过程提供了机制见解。
更新日期:2024-06-12
中文翻译:

DDM1结合核小体结构揭示异染色质重塑机制
SWI/SNF2 染色质重塑因子降低 DNA 甲基化 1 (DDM1) 对于常染色质和异染色质区域转座元件 (TE) 的沉默至关重要。在这里,我们在 ATP 类似物 ADP-BeFx 存在的情况下以近原子分辨率测定了 DDM1-核小体和 DDM1-核小体复合物的冷冻电镜结构。该结构表明,与含有组蛋白 H2A.W 的组蛋白八聚体相比,核小体 DNA 在含有组蛋白 H2A 的组蛋白八聚体的表面上更多地展开。 DDM1 包含核小体的一个 DNA 环,并与组蛋白 H4 的 N 末端尾部相互作用。尽管我们没有在我们的结构中观察到 DDM1-H2A.W 相互作用,但下拉实验的结果表明 DDM1 和组蛋白 H2A.W 核心区域之间存在直接相互作用。我们的工作为植物中由 DDM1 驱动的异染色质重塑过程提供了机制见解。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号