当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pd-catalyzed relay Heck arylation of alkenyl alcohols with arylsulfonium salts
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-12 , DOI: 10.1039/d4qo00618f Jia-Wei Song 1 , Fang Xia 1 , Xiu-Lan Zhang 1 , Cheng-Pan Zhang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-12 , DOI: 10.1039/d4qo00618f Jia-Wei Song 1 , Fang Xia 1 , Xiu-Lan Zhang 1 , Cheng-Pan Zhang 1
Affiliation
|
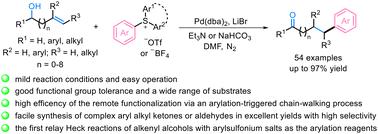
A palladium-catalyzed tandem Heck arylation and isomerization of alkenyl alcohols using arylsulfonium salts is reported. The reaction enabled facile conversion of various allylic and non-allylic alcohols to the corresponding arylalkyl ketones or aldehydes in good to excellent yields. Different types of arylsulfonium salts were verified as powerful arylation reagents in the relay Heck reactions via chain-walking without being affected by the sulfide byproducts. The transformation exhibited obvious advantages such as high efficiency, mild conditions, good functional group tolerance, excellent regioselectivity, and a wide range of substrates, which allowed remote functionalization of alkenyl alcohols with more complex aryl transfer reagents. A plausible reaction mechanism was presumed to involve the initial Heck arylation of the alkenyl moiety with arylsulfonium salts, repeated migratory insertion and β-hydride elimination, and enol isomerization to yield the final product. Moreover, the Pd-catalyst remains coordinated to the substrate during the relay process. This work represents the first remote functionalization of alkenyl alcohols with arylsulfonium salts as the arylation reagents.
中文翻译:

Pd 催化烯基醇与芳基锍盐的中继 Heck 芳基化反应
报道了使用芳基锍盐进行的钯催化串联 Heck 芳基化和烯基醇异构化。该反应能够将各种烯丙醇和非烯丙醇轻松转化为相应的芳烷基酮或醛,收率良好至优异。不同类型的芳基锍盐被证实在通过链行走的中继 Heck 反应中是强大的芳基化试剂,且不受硫化物副产物的影响。该转化表现出效率高、条件温和、官能团耐受性好、区域选择性好、底物范围广等明显优点,可以使用更复杂的芳基转移试剂对烯醇进行远程官能化。推测合理的反应机制涉及烯基部分与芳基锍盐的初始 Heck 芳基化、重复迁移插入和 β-氢化物消除以及烯醇异构化以产生最终产物。此外,在中继过程中,钯催化剂仍与基底保持配位。这项工作代表了首次使用芳基锍盐作为芳基化试剂对烯基醇进行远程官能化。
更新日期:2024-06-12
中文翻译:

Pd 催化烯基醇与芳基锍盐的中继 Heck 芳基化反应
报道了使用芳基锍盐进行的钯催化串联 Heck 芳基化和烯基醇异构化。该反应能够将各种烯丙醇和非烯丙醇轻松转化为相应的芳烷基酮或醛,收率良好至优异。不同类型的芳基锍盐被证实在通过链行走的中继 Heck 反应中是强大的芳基化试剂,且不受硫化物副产物的影响。该转化表现出效率高、条件温和、官能团耐受性好、区域选择性好、底物范围广等明显优点,可以使用更复杂的芳基转移试剂对烯醇进行远程官能化。推测合理的反应机制涉及烯基部分与芳基锍盐的初始 Heck 芳基化、重复迁移插入和 β-氢化物消除以及烯醇异构化以产生最终产物。此外,在中继过程中,钯催化剂仍与基底保持配位。这项工作代表了首次使用芳基锍盐作为芳基化试剂对烯基醇进行远程官能化。






































 京公网安备 11010802027423号
京公网安备 11010802027423号