当前位置:
X-MOL 学术
›
Environ. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative proteomics of a versatile, marine, iron-oxidizing chemolithoautotroph
Environmental Microbiology ( IF 4.3 ) Pub Date : 2024-06-11 , DOI: 10.1111/1462-2920.16632 Roman A. Barco 1, 2, 3 , N. Merino 1, 4, 5 , B. Lam 2 , B. Budnik 6 , M. Kaplan 7 , F. Wu 8 , J. P. Amend 1, 2 , K. H. Nealson 1, 2 , D. Emerson 3
Environmental Microbiology ( IF 4.3 ) Pub Date : 2024-06-11 , DOI: 10.1111/1462-2920.16632 Roman A. Barco 1, 2, 3 , N. Merino 1, 4, 5 , B. Lam 2 , B. Budnik 6 , M. Kaplan 7 , F. Wu 8 , J. P. Amend 1, 2 , K. H. Nealson 1, 2 , D. Emerson 3
Affiliation
|
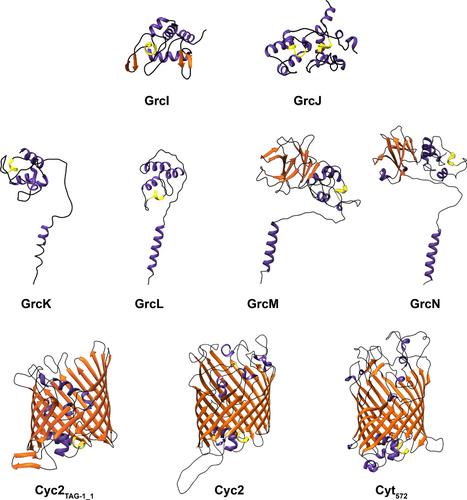
This study conducted a comparative proteomic analysis to identify potential genetic markers for the biological function of chemolithoautotrophic iron oxidation in the marine bacterium Ghiorsea bivora. To date, this is the only characterized species in the class Zetaproteobacteria that is not an obligate iron-oxidizer, providing a unique opportunity to investigate differential protein expression to identify key genes involved in iron-oxidation at circumneutral pH. Over 1000 proteins were identified under both iron- and hydrogen-oxidizing conditions, with differentially expressed proteins found in both treatments. Notably, a gene cluster upregulated during iron oxidation was identified. This cluster contains genes encoding for cytochromes that share sequence similarity with the known iron-oxidase, Cyc2. Interestingly, these cytochromes, conserved in both Bacteria and Archaea, do not exhibit the typical β-barrel structure of Cyc2. This cluster potentially encodes a biological nanowire-like transmembrane complex containing multiple redox proteins spanning the inner membrane, periplasm, outer membrane, and extracellular space. The upregulation of key genes associated with this complex during iron-oxidizing conditions was confirmed by quantitative reverse transcription-PCR. These findings were further supported by electromicrobiological methods, which demonstrated negative current production by G. bivora in a three-electrode system poised at a cathodic potential. This research provides significant insights into the biological function of chemolithoautotrophic iron oxidation.
中文翻译:

多功能海洋铁氧化化能自养生物的比较蛋白质组学
本研究进行了比较蛋白质组分析,以确定海洋细菌 Ghiorsea bivora 化能自养铁氧化生物学功能的潜在遗传标记。迄今为止,这是 Zetaproteobacteria 中唯一一个非专性铁氧化剂的特征物种,为研究差异蛋白表达以识别中性 pH 条件下参与铁氧化的关键基因提供了独特的机会。在铁氧化和氢氧化条件下均鉴定出超过 1000 种蛋白质,并且在两种处理中都发现了差异表达的蛋白质。值得注意的是,发现了铁氧化过程中上调的基因簇。该簇包含编码细胞色素的基因,这些基因与已知的铁氧化酶 Cyc2 具有序列相似性。有趣的是,这些细胞色素在细菌和古细菌中都保守,但并不表现出 Cyc2 典型的 β 桶结构。该簇可能编码一种生物纳米线样跨膜复合物,包含跨越内膜、周质、外膜和细胞外空间的多种氧化还原蛋白。通过定量逆转录 PCR 证实了铁氧化条件下与该复合物相关的关键基因的上调。这些发现得到了电微生物学方法的进一步支持,该方法证明了 G. bivora 在处于阴极电位的三电极系统中产生负电流。这项研究为化学自养铁氧化的生物学功能提供了重要的见解。
更新日期:2024-06-11
中文翻译:

多功能海洋铁氧化化能自养生物的比较蛋白质组学
本研究进行了比较蛋白质组分析,以确定海洋细菌 Ghiorsea bivora 化能自养铁氧化生物学功能的潜在遗传标记。迄今为止,这是 Zetaproteobacteria 中唯一一个非专性铁氧化剂的特征物种,为研究差异蛋白表达以识别中性 pH 条件下参与铁氧化的关键基因提供了独特的机会。在铁氧化和氢氧化条件下均鉴定出超过 1000 种蛋白质,并且在两种处理中都发现了差异表达的蛋白质。值得注意的是,发现了铁氧化过程中上调的基因簇。该簇包含编码细胞色素的基因,这些基因与已知的铁氧化酶 Cyc2 具有序列相似性。有趣的是,这些细胞色素在细菌和古细菌中都保守,但并不表现出 Cyc2 典型的 β 桶结构。该簇可能编码一种生物纳米线样跨膜复合物,包含跨越内膜、周质、外膜和细胞外空间的多种氧化还原蛋白。通过定量逆转录 PCR 证实了铁氧化条件下与该复合物相关的关键基因的上调。这些发现得到了电微生物学方法的进一步支持,该方法证明了 G. bivora 在处于阴极电位的三电极系统中产生负电流。这项研究为化学自养铁氧化的生物学功能提供了重要的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号