当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expedient Synthesis of Thermally Stable Acyclic Amino(haloaryl)carbenes: Experimental and Theoretical Evidence of “Push–Pull” Stabilized Carbenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-10 , DOI: 10.1021/jacs.4c04872 Damien Magis 1 , Jorge Juan Cabrera-Trujillo 2 , Joan Vignolle 3 , Jean-Marc Sotiropoulos 2 , Daniel Taton 3 , Karinne Miqueu 2 , Yannick Landais 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-10 , DOI: 10.1021/jacs.4c04872 Damien Magis 1 , Jorge Juan Cabrera-Trujillo 2 , Joan Vignolle 3 , Jean-Marc Sotiropoulos 2 , Daniel Taton 3 , Karinne Miqueu 2 , Yannick Landais 1
Affiliation
|
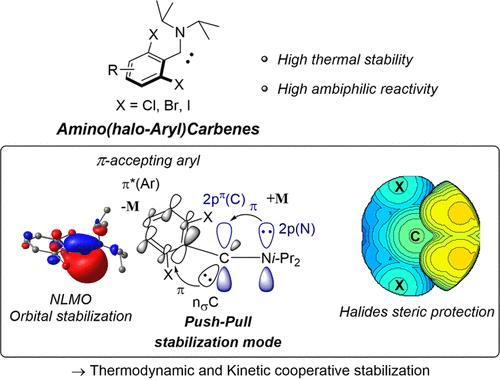
A library of novel structurally related singlet carbenes, namely, acyclic amino(haloaryl)carbenes, was designed by a high-yielding two-step procedure, and their chemical stability explored both experimentally and theoretically. Thanks to a careful selection of both the amino and the aryl substitution pattern, these carbenes exhibit a wide range of stability and reactivity, spanning from rapid self-dimerization for carbenes featuring ortho-F substituents to very high chemical stability as bare carbenes, up to 60 °C for several hours for compounds carrying ortho-Br substituents. Their structure was determined through NMR and X-ray diffraction studies, and their reactivity evaluated in benchmark reactions, highlighting the ambiphilic character of this novel class of singlet carbenes. In contrast with previously reported aryl substituents incorporating o-CF3 and t-Bu groups, which were considered “spectator”, the high chemical stability of some of these carbenes relates to the stabilization of the σ-orbital of the carbene center by the π-accepting haloaryl substituent through delocalization. Kinetic protection of the carbene center is also provided by the ortho-halogen atoms, as demonstrated computationally. This push–pull stabilization effect makes acyclic amino(haloaryl) carbenes among the most ambiphilic stable carbenes reported to date, holding promise for a variety of applications.
中文翻译:

热稳定无环氨基(卤芳基)卡宾的便捷合成:“推拉”稳定卡宾的实验和理论证据
通过高产两步程序设计了一个新型结构相关的单线态卡宾库,即无环氨基(卤代芳基)卡宾,并从实验和理论上探索了它们的化学稳定性。由于仔细选择了氨基和芳基取代模式,这些卡宾表现出广泛的稳定性和反应性,从具有邻位-F取代基的卡宾的快速自二聚到作为裸卡宾的非常高的化学稳定性,高达对于带有邻位-Br 取代基的化合物,60 °C 几个小时。它们的结构是通过核磁共振和 X 射线衍射研究确定的,并在基准反应中评估了它们的反应性,突出了这类新型单线态卡宾的两亲性特征。与之前报道的包含o -CF 3和t -Bu 基团的芳基取代基(被认为是“旁观者”)相比,其中一些卡宾的高化学稳定性与 π 对卡宾中心 σ 轨道的稳定有关。 -通过离域接受卤代芳基取代基。正如计算所证明的那样,邻位卤素原子也提供了卡宾中心的动力学保护。这种推拉稳定效应使无环氨基(卤代芳基)卡宾成为迄今为止报道的两亲性最稳定的卡宾之一,有望在各种应用中发挥作用。
更新日期:2024-06-10
中文翻译:

热稳定无环氨基(卤芳基)卡宾的便捷合成:“推拉”稳定卡宾的实验和理论证据
通过高产两步程序设计了一个新型结构相关的单线态卡宾库,即无环氨基(卤代芳基)卡宾,并从实验和理论上探索了它们的化学稳定性。由于仔细选择了氨基和芳基取代模式,这些卡宾表现出广泛的稳定性和反应性,从具有邻位-F取代基的卡宾的快速自二聚到作为裸卡宾的非常高的化学稳定性,高达对于带有邻位-Br 取代基的化合物,60 °C 几个小时。它们的结构是通过核磁共振和 X 射线衍射研究确定的,并在基准反应中评估了它们的反应性,突出了这类新型单线态卡宾的两亲性特征。与之前报道的包含o -CF 3和t -Bu 基团的芳基取代基(被认为是“旁观者”)相比,其中一些卡宾的高化学稳定性与 π 对卡宾中心 σ 轨道的稳定有关。 -通过离域接受卤代芳基取代基。正如计算所证明的那样,邻位卤素原子也提供了卡宾中心的动力学保护。这种推拉稳定效应使无环氨基(卤代芳基)卡宾成为迄今为止报道的两亲性最稳定的卡宾之一,有望在各种应用中发挥作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号