当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Allylation of Endocyclic 1-Azaallyl Anions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-10 , DOI: 10.1021/acs.joc.4c00743 Xiaoyu Yang 1 , Biao Zhang 1 , Junhao Ruan 1 , Kaining Duanmu 1 , Weijie Chen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-06-10 , DOI: 10.1021/acs.joc.4c00743 Xiaoyu Yang 1 , Biao Zhang 1 , Junhao Ruan 1 , Kaining Duanmu 1 , Weijie Chen 1
Affiliation
|
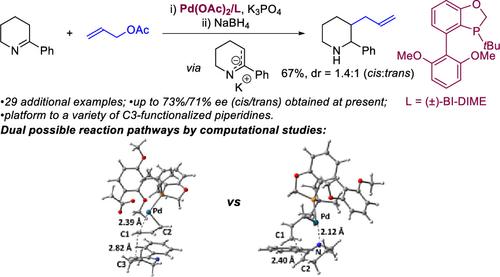
Endocyclic 1-azaallyl anions engage allyl acetates in a palladium-catalyzed allylation followed by reduction to give unprotected 2-(hetero)aryl-3-allylpiperidines and 2-allyl-3-arylmorpholines, products not easily accessible by other means. The allyl group is then readily transformed into a variety of functional groups. Preliminary studies on the asymmetric variant of the reaction using an enantiomerically pure BI-DIME-type ligand provide the product with moderate enantioselectivity. Computational studies suggest that energy barriers of inner-sphere reductive elimination and outer-sphere nucleophilic substitution are almost the same, which makes both of them possible reaction pathways. In addition, the inner-sphere mechanism displays an enantiodiscriminating C–C bond forming step, while the outer-sphere mechanism is much less selective, which combined to give the asymmetric variant of the reaction moderate enantioselectivity.
中文翻译:

钯催化环内 1-氮杂烯丙基阴离子的烯丙基化
环内 1-氮杂烯丙基阴离子在钯催化的烯丙基化中与乙酸烯丙酯结合,然后还原,得到未保护的 2-(杂)芳基-3-烯丙基哌啶和 2-烯丙基-3-芳基吗啉,这些产品不易通过其他方式获得。然后烯丙基很容易转化成各种官能团。使用对映体纯的 BI-DIME 型配体对反应的不对称变体进行的初步研究表明,产物具有中等的对映选择性。计算研究表明,内层还原消除和外层亲核取代的能垒几乎相同,这使得它们都是可能的反应途径。此外,内球机制显示出对映体区别性的C-C键形成步骤,而外球机制的选择性要低得多,这两者结合起来使反应的不对称变体具有中等对映选择性。
更新日期:2024-06-10
中文翻译:

钯催化环内 1-氮杂烯丙基阴离子的烯丙基化
环内 1-氮杂烯丙基阴离子在钯催化的烯丙基化中与乙酸烯丙酯结合,然后还原,得到未保护的 2-(杂)芳基-3-烯丙基哌啶和 2-烯丙基-3-芳基吗啉,这些产品不易通过其他方式获得。然后烯丙基很容易转化成各种官能团。使用对映体纯的 BI-DIME 型配体对反应的不对称变体进行的初步研究表明,产物具有中等的对映选择性。计算研究表明,内层还原消除和外层亲核取代的能垒几乎相同,这使得它们都是可能的反应途径。此外,内球机制显示出对映体区别性的C-C键形成步骤,而外球机制的选择性要低得多,这两者结合起来使反应的不对称变体具有中等对映选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号