当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Route Design and Scale-Up of a Topoisomerase I Inhibitor Antibody–Drug Conjugate Payload
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-11 , DOI: 10.1021/acs.oprd.4c00175 William R. F. Goundry 1 , Andrew M. Poulton 1 , Matthew Welham 2 , Kuangchu Dai 3 , Xiaohong Zhu 3 , Haijun Tao 3 , Canlin Tang 3 , Fanny Magne 4 , Bertrand Cottineau 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-11 , DOI: 10.1021/acs.oprd.4c00175 William R. F. Goundry 1 , Andrew M. Poulton 1 , Matthew Welham 2 , Kuangchu Dai 3 , Xiaohong Zhu 3 , Haijun Tao 3 , Canlin Tang 3 , Fanny Magne 4 , Bertrand Cottineau 4
Affiliation
|
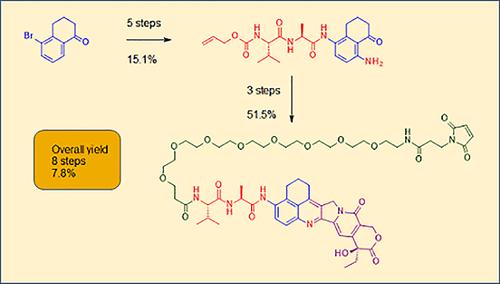
AstraZeneca is currently developing an antibody–drug conjugate for the treatment of cancer with a topoisomerase I inhibitor payload. The drug portion of the molecule is an analogue of the natural product camptothecin. We describe the initial scale-up of the synthesis to meet preclinical timelines, including route design work to shorten the route by five steps. We also detail several problems we encountered, most notably a low-yield final step. We developed a second-generation route to address these issues, increasing the overall yield from 3.4% to 7.8% while reducing the process mass intensity by 66%. We discuss the impurities formed throughout the process and highlight our workup and purification strategy to remove them.
中文翻译:

拓扑异构酶 I 抑制剂抗体-药物偶联物有效负载的路线设计和放大
阿斯利康目前正在开发一种抗体-药物偶联物,用于通过拓扑异构酶 I 抑制剂有效负载治疗癌症。该分子的药物部分是天然产物喜树碱的类似物。我们描述了合成的初步放大以满足临床前时间表,包括将路线缩短五个步骤的路线设计工作。我们还详细介绍了我们遇到的几个问题,最明显的是最后一步的低产量。我们开发了第二代路线来解决这些问题,将总产量从 3.4% 提高到 7.8%,同时将工艺质量强度降低 66%。我们讨论了整个过程中形成的杂质,并重点介绍了去除它们的后处理和纯化策略。
更新日期:2024-06-11
中文翻译:

拓扑异构酶 I 抑制剂抗体-药物偶联物有效负载的路线设计和放大
阿斯利康目前正在开发一种抗体-药物偶联物,用于通过拓扑异构酶 I 抑制剂有效负载治疗癌症。该分子的药物部分是天然产物喜树碱的类似物。我们描述了合成的初步放大以满足临床前时间表,包括将路线缩短五个步骤的路线设计工作。我们还详细介绍了我们遇到的几个问题,最明显的是最后一步的低产量。我们开发了第二代路线来解决这些问题,将总产量从 3.4% 提高到 7.8%,同时将工艺质量强度降低 66%。我们讨论了整个过程中形成的杂质,并重点介绍了去除它们的后处理和纯化策略。






































 京公网安备 11010802027423号
京公网安备 11010802027423号