当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dearomative difunctionalization of arenes via highly selective radical relay reactions
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-11 , DOI: 10.1039/d4qo00964a Ning Lei 1 , Qian Zhang 2 , Pan Tao 1 , Cong Lu 1 , Qian Lei 3 , Ke Zheng 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-11 , DOI: 10.1039/d4qo00964a Ning Lei 1 , Qian Zhang 2 , Pan Tao 1 , Cong Lu 1 , Qian Lei 3 , Ke Zheng 1
Affiliation
|
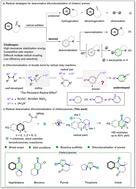
Dearomatization of arenes emerges as a reliable strategy for crafting intricate 3D polycyclic frameworks. Nonetheless, the development of an efficient method for dearomatization across diverse arenes remains a formidable challenge, particularly in the case of unactivated benzene. In this study, we present a facile dearomative difunctionalization approach for unactivated arenes through a highly selective radical relay reaction. The reaction operates under mild conditions, devoid of metals, photocatalysts, and additives. This method exhibits a broad substrate scope and exceptional functional group tolerance, successfully transforming various aromatics, including inert naphthalene and benzene rings, indoles, pyrroles, benzofuran, and thiophene. Bioactivity assessments reveal a significant inhibitory effect of the synthesized complex polycyclic frameworks on HCT 116 cancer cells, with subsequent mouse experiments providing compelling evidence of its feasibility.
中文翻译:

通过高选择性自由基接力反应进行芳烃的脱芳香双官能化
芳烃的脱芳构化成为构建复杂 3D 多环框架的可靠策略。尽管如此,开发一种有效的跨不同芳烃脱芳构化方法仍然是一个艰巨的挑战,特别是在未活化的苯的情况下。在这项研究中,我们通过高度选择性的自由基中继反应,提出了一种针对未活化芳烃的简便的脱芳香双官能化方法。该反应在温和条件下进行,不含金属、光催化剂和添加剂。该方法具有广泛的底物范围和出色的官能团耐受性,成功转化了各种芳香族化合物,包括惰性萘和苯环、吲哚、吡咯、苯并呋喃和噻吩。生物活性评估揭示了合成的复杂多环框架对 HCT 116 癌细胞具有显着的抑制作用,随后的小鼠实验为其可行性提供了令人信服的证据。
更新日期:2024-06-11
中文翻译:

通过高选择性自由基接力反应进行芳烃的脱芳香双官能化
芳烃的脱芳构化成为构建复杂 3D 多环框架的可靠策略。尽管如此,开发一种有效的跨不同芳烃脱芳构化方法仍然是一个艰巨的挑战,特别是在未活化的苯的情况下。在这项研究中,我们通过高度选择性的自由基中继反应,提出了一种针对未活化芳烃的简便的脱芳香双官能化方法。该反应在温和条件下进行,不含金属、光催化剂和添加剂。该方法具有广泛的底物范围和出色的官能团耐受性,成功转化了各种芳香族化合物,包括惰性萘和苯环、吲哚、吡咯、苯并呋喃和噻吩。生物活性评估揭示了合成的复杂多环框架对 HCT 116 癌细胞具有显着的抑制作用,随后的小鼠实验为其可行性提供了令人信服的证据。






































 京公网安备 11010802027423号
京公网安备 11010802027423号