当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitative comparison of the copolymerisation kinetics in catalyst-transfer copolymerisation to synthesise polythiophenes
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-06-10 , DOI: 10.1039/d4py00009a Yifei He 1 , Christine K. Luscombe 2
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-06-10 , DOI: 10.1039/d4py00009a Yifei He 1 , Christine K. Luscombe 2
Affiliation
|
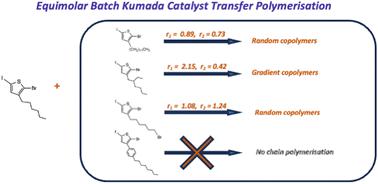
Polythiophenes are one of the most widely studied conjugated polymers. With the discovery of the chain mechanism of Kumada catalyst-transfer polymerisation (KCTP), various polythiophene copolymer structures, such as random, block, and gradient copolymers, have been synthesized via batch or semi-batch (sequential addition) methods. However, the lack of quantitative kinetic data for thiophene monomers brings challenges to experimental design and structure prediction when synthesizing the copolymers. In this study, the reactivity ratios and the polymerisation rate constants of 3-hexylthiophene with 4 thiophene comonomers in KCTP are measured by adapting the Mayo–Lewis equation and the first-order kinetic behaviour of chain polymerisation. The obtained kinetic information highlights the impact of the monomer structure on the reactivity in the copolymerisations. The kinetic data are used to predict the copolymer structure of equimolar batch copolymerisations of the 4 thiophene derivatives with 3-hexylthiophene, with the experimental data agreeing well with the predictions. 3-Dodecylthiophene and 3-(6-bromo)hexylthiophene, which have higher structural similarity to 3-hexylthiophene, show nearly equivalent reactivity to 3-hexylthiophene and give random copolymers in the batch copolymerisation. 3-(2-Ethylhexyl)thiophene with a branched side chain is less reactive compared to 3-hexylthiophene and failed to homopolymerize at room temperature, but produced gradient copolymers with 3-hexylthiophene. Finally, the bulkiest 3-(4-octylphenyl)thiophene, despite its ability to homopolymerize, failed to maintain chain polymerisation in the copolymerisation with 3-hexylthiophene, possibly due to the large steric hindrance caused by the phenyl ring directly attached to the thiophene center. This study highlights the importance of monomer structures in copolymerisations and the need for accurate kinetic data.
中文翻译:

催化剂转移共聚合成聚噻吩共聚动力学的定量比较
聚噻吩是研究最广泛的共轭聚合物之一。随着熊田催化剂转移聚合(KCTP)链机制的发现,各种聚噻吩共聚物结构,如无规、嵌段和梯度共聚物,已经通过间歇或半间歇(顺序添加)方法合成。然而,噻吩单体定量动力学数据的缺乏给合成共聚物时的实验设计和结构预测带来了挑战。在本研究中,通过采用 Mayo-Lewis 方程和链聚合的一级动力学行为来测量 KCTP 中 3-己基噻吩与 4 个噻吩共聚单体的反应活性比和聚合速率常数。获得的动力学信息强调了单体结构对共聚反应活性的影响。利用动力学数据预测了4种噻吩衍生物与3-己基噻吩等摩尔分批共聚的共聚物结构,实验数据与预测吻合良好。 3-十二烷基噻吩和3-(6-溴)己基噻吩与3-己基噻吩具有更高的结构相似性,表现出与3-己基噻吩几乎相同的反应性,并且在间歇共聚中产生无规共聚物。与3-己基噻吩相比,具有支化侧链的3-(2-乙基己基)噻吩的反应性较低,并且在室温下不能均聚,但与3-己基噻吩产生梯度共聚物。最后,体积最大的3-(4-辛基苯基)噻吩尽管具有均聚能力,但在与3-己基噻吩的共聚中未能保持链聚合,这可能是由于直接连接到噻吩中心的苯环造成的大空间位阻。 这项研究强调了共聚中单体结构的重要性以及对准确动力学数据的需求。
更新日期:2024-06-10
中文翻译:

催化剂转移共聚合成聚噻吩共聚动力学的定量比较
聚噻吩是研究最广泛的共轭聚合物之一。随着熊田催化剂转移聚合(KCTP)链机制的发现,各种聚噻吩共聚物结构,如无规、嵌段和梯度共聚物,已经通过间歇或半间歇(顺序添加)方法合成。然而,噻吩单体定量动力学数据的缺乏给合成共聚物时的实验设计和结构预测带来了挑战。在本研究中,通过采用 Mayo-Lewis 方程和链聚合的一级动力学行为来测量 KCTP 中 3-己基噻吩与 4 个噻吩共聚单体的反应活性比和聚合速率常数。获得的动力学信息强调了单体结构对共聚反应活性的影响。利用动力学数据预测了4种噻吩衍生物与3-己基噻吩等摩尔分批共聚的共聚物结构,实验数据与预测吻合良好。 3-十二烷基噻吩和3-(6-溴)己基噻吩与3-己基噻吩具有更高的结构相似性,表现出与3-己基噻吩几乎相同的反应性,并且在间歇共聚中产生无规共聚物。与3-己基噻吩相比,具有支化侧链的3-(2-乙基己基)噻吩的反应性较低,并且在室温下不能均聚,但与3-己基噻吩产生梯度共聚物。最后,体积最大的3-(4-辛基苯基)噻吩尽管具有均聚能力,但在与3-己基噻吩的共聚中未能保持链聚合,这可能是由于直接连接到噻吩中心的苯环造成的大空间位阻。 这项研究强调了共聚中单体结构的重要性以及对准确动力学数据的需求。












































 京公网安备 11010802027423号
京公网安备 11010802027423号