当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The dissolution behavior of 3,4-O-isopropylidene clindamycin in twelve mono-solvents: Solubility, intermolecular interactions, and apparent thermodynamics
Thermochimica Acta ( IF 3.1 ) Pub Date : 2024-06-02 , DOI: 10.1016/j.tca.2024.179791 Huanxin Li , Bo Zhu , Kenan Sun , Xin Ding
Thermochimica Acta ( IF 3.1 ) Pub Date : 2024-06-02 , DOI: 10.1016/j.tca.2024.179791 Huanxin Li , Bo Zhu , Kenan Sun , Xin Ding
|
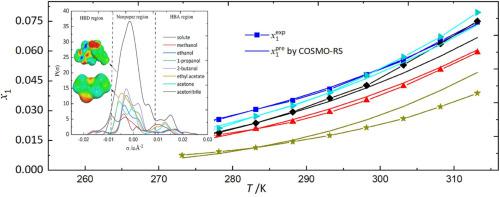
The solubility of 3, 4--isopropylidene clindamycin (OIC) was determined in twelve solvents at = 101.3 kPa with the temperature from 273.15 K to 313.15 K. The solubility of OIC increases with the raise temperature. Among the selected solvents, 2-butanol showed the best dissolving ability for OIC, while acetonitrile showed the worst dissolving ability. The obtained data are neatly correlated with five models, including van't Hoff, Yaws, , Wilson, and nonrandom two-liquid interaction model (NRTL), with Yaws equation yielding the most accurate predicted results. Moreover, the Conductor-like Screening Model for Real solvents (COSMO-RS) provides better calculated results for the protic solvents than the aprotic ones except for acetonitrile. The dissolving mechanism of OIC was investigated with intermolecular interaction between OIC and solvents, and the analyses indicate that the dissolving process is affected by intermolecular interaction, molecular shape, and size of both solvents and the solute. The apparent thermodynamics analysis shows that the dissolving process of OIC before reaching equilibrium in the twelve solvents is spontaneous, endothermic, and enthalpy driven.
中文翻译:

3,4-O-异丙叉克林霉素在十二种单溶剂中的溶解行为:溶解度、分子间相互作用和表观热力学
测定了3, 4-亚异丙基克林霉素(OIC)在12种溶剂中的溶解度,压力=101.3 kPa,温度为273.15 K至313.15 K。OIC的溶解度随着温度的升高而增加。在所选溶剂中,2-丁醇对OIC的溶解能力最好,而乙腈的溶解能力最差。获得的数据与 van't Hoff、Yaws、Wilson 和非随机二液相互作用模型 (NRTL) 等 5 个模型完美相关,其中 Yaws 方程的预测结果最为准确。此外,真实溶剂的类导体筛选模型 (COSMO-RS) 为质子溶剂提供了比除乙腈以外的非质子溶剂更好的计算结果。通过OIC与溶剂之间的分子间相互作用研究了OIC的溶解机理,分析表明溶解过程受到分子间相互作用、分子形状以及溶剂和溶质的尺寸的影响。表观热力学分析表明,OIC 在 12 种溶剂中达到平衡之前的溶解过程是自发的、吸热的和焓驱动的。
更新日期:2024-06-02
中文翻译:

3,4-O-异丙叉克林霉素在十二种单溶剂中的溶解行为:溶解度、分子间相互作用和表观热力学
测定了3, 4-亚异丙基克林霉素(OIC)在12种溶剂中的溶解度,压力=101.3 kPa,温度为273.15 K至313.15 K。OIC的溶解度随着温度的升高而增加。在所选溶剂中,2-丁醇对OIC的溶解能力最好,而乙腈的溶解能力最差。获得的数据与 van't Hoff、Yaws、Wilson 和非随机二液相互作用模型 (NRTL) 等 5 个模型完美相关,其中 Yaws 方程的预测结果最为准确。此外,真实溶剂的类导体筛选模型 (COSMO-RS) 为质子溶剂提供了比除乙腈以外的非质子溶剂更好的计算结果。通过OIC与溶剂之间的分子间相互作用研究了OIC的溶解机理,分析表明溶解过程受到分子间相互作用、分子形状以及溶剂和溶质的尺寸的影响。表观热力学分析表明,OIC 在 12 种溶剂中达到平衡之前的溶解过程是自发的、吸热的和焓驱动的。































 京公网安备 11010802027423号
京公网安备 11010802027423号