当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual-targeted nanoparticulate drug delivery systems for enhancing triple-negative breast cancer treatment
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.jconrel.2024.06.012 Shunzhe Zheng 1 , Meng Li 1 , Wenqian Xu 1 , Jiaxin Zhang 1 , Guanting Li 1 , Hongying Xiao 1 , Xinying Liu 1 , Jianbin Shi 1 , Fengli Xia 1 , Chutong Tian 2 , Ken-Ichiro Kamei 3
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.jconrel.2024.06.012 Shunzhe Zheng 1 , Meng Li 1 , Wenqian Xu 1 , Jiaxin Zhang 1 , Guanting Li 1 , Hongying Xiao 1 , Xinying Liu 1 , Jianbin Shi 1 , Fengli Xia 1 , Chutong Tian 2 , Ken-Ichiro Kamei 3
Affiliation
|
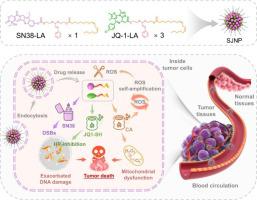
The efficacy of DNA-damaging agents, such as the topoisomerase I inhibitor SN38, is often compromised by the robust DNA repair mechanisms in tumor cells, notably homologous recombination (HR) repair. Addressing this challenge, we introduce a novel nano-strategy utilizing binary tumor-killing mechanisms to enhance the therapeutic impact of DNA damage and mitochondrial dysfunction in cancer treatment. Our approach employs a synergistic drug pair comprising SN38 and the BET inhibitor JQ-1. We synthesized two prodrugs by conjugating linoleic acid (LA) to SN38 and JQ-1 via a cinnamaldehyde thioacetal (CT) bond, facilitating co-delivery. These prodrugs co-assemble into a nanostructure, referred to as SJNP, in an optimal synergistic ratio. SJNP was validated for its efficacy at both the cellular and tissue levels, where it primarily disrupts the transcription factor protein BRD4. This disruption leads to downregulation of BRCA1 and RAD51, impairing the HR process and exacerbating DNA damage. Additionally, SJNP releases cinnamaldehyde (CA) upon CT linkage cleavage, elevating intracellular ROS levels in a self-amplifying manner and inducing ROS-mediated mitochondrial dysfunction. Our results indicate that SJNP effectively targets murine triple-negative breast cancer (TNBC) with minimal adverse toxicity, showcasing its potential as a formidable opponent in the fight against cancer.
中文翻译:

用于增强三阴性乳腺癌治疗的双靶向纳米颗粒药物递送系统
DNA 损伤剂(例如拓扑异构酶 I 抑制剂 SN38)的功效常常受到肿瘤细胞中强大的 DNA 修复机制(特别是同源重组 (HR) 修复)的影响。为了应对这一挑战,我们引入了一种新颖的纳米策略,利用二元肿瘤杀伤机制来增强 DNA 损伤和线粒体功能障碍在癌症治疗中的治疗效果。我们的方法采用包含 SN38 和 BET 抑制剂 JQ-1 的协同药物对。我们通过肉桂醛硫缩醛 (CT) 键将亚油酸 (LA) 与 SN38 和 JQ-1 缀合,从而促进共同递送,从而合成了两种前药。这些前药以最佳协同比例共同组装成纳米结构,称为 SJNP。 SJNP 的功效在细胞和组织水平上得到了验证,它主要破坏转录因子蛋白 BRD4。这种破坏导致 BRCA1 和 RAD51 下调,损害 HR 过程并加剧 DNA 损伤。此外,SJNP 在 CT 连接断裂时释放肉桂醛 (CA),以自我放大的方式提高细胞内 ROS 水平并诱导 ROS 介导的线粒体功能障碍。我们的结果表明,SJNP 能有效靶向小鼠三阴性乳腺癌 (TNBC),且不良毒性最小,显示出其作为抗癌强大对手的潜力。
更新日期:2024-06-08
中文翻译:

用于增强三阴性乳腺癌治疗的双靶向纳米颗粒药物递送系统
DNA 损伤剂(例如拓扑异构酶 I 抑制剂 SN38)的功效常常受到肿瘤细胞中强大的 DNA 修复机制(特别是同源重组 (HR) 修复)的影响。为了应对这一挑战,我们引入了一种新颖的纳米策略,利用二元肿瘤杀伤机制来增强 DNA 损伤和线粒体功能障碍在癌症治疗中的治疗效果。我们的方法采用包含 SN38 和 BET 抑制剂 JQ-1 的协同药物对。我们通过肉桂醛硫缩醛 (CT) 键将亚油酸 (LA) 与 SN38 和 JQ-1 缀合,从而促进共同递送,从而合成了两种前药。这些前药以最佳协同比例共同组装成纳米结构,称为 SJNP。 SJNP 的功效在细胞和组织水平上得到了验证,它主要破坏转录因子蛋白 BRD4。这种破坏导致 BRCA1 和 RAD51 下调,损害 HR 过程并加剧 DNA 损伤。此外,SJNP 在 CT 连接断裂时释放肉桂醛 (CA),以自我放大的方式提高细胞内 ROS 水平并诱导 ROS 介导的线粒体功能障碍。我们的结果表明,SJNP 能有效靶向小鼠三阴性乳腺癌 (TNBC),且不良毒性最小,显示出其作为抗癌强大对手的潜力。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号