当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
First Multikilogram Synthesis of the Next-Generation Oral Selective ERα Degrader Camizestrant
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-07 , DOI: 10.1021/acs.oprd.4c00135 Matthew R. Tatton 1 , Gordon S. Currie 1 , Bradley Adams 1 , Carl-Johan Aurell 2 , Karl Broberg 1 , Andrew D. Campbell 3 , Kuangchu Dai 4 , Marcus Malmgren 2 , Andrew Ikin 1 , Sophie L. M. Janbon 1 , Martin Sims 1 , Joanna Hemming-Taylor 1 , Victoria Winterbottom 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-07 , DOI: 10.1021/acs.oprd.4c00135 Matthew R. Tatton 1 , Gordon S. Currie 1 , Bradley Adams 1 , Carl-Johan Aurell 2 , Karl Broberg 1 , Andrew D. Campbell 3 , Kuangchu Dai 4 , Marcus Malmgren 2 , Andrew Ikin 1 , Sophie L. M. Janbon 1 , Martin Sims 1 , Joanna Hemming-Taylor 1 , Victoria Winterbottom 1
Affiliation
|
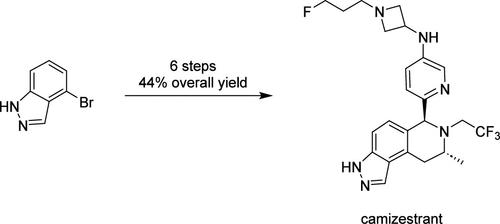
Camizestrant is currently being investigated in multiple Phase 3 clinical trials for ER+ breast cancer. This article describes our efforts toward the first manufacture of clinical material. Strategic process development focused on delivering robust processes and control points that could be scaled to deliver kilograms of material of the right quality and meet expedited project timelines. Highlights include optimization of an efficient Buchwald–Hartwig amination, development of a diastereoselective Pictet–Spengler reaction followed by an efficient isolation, and a significant reduction in the number of chromatography stages from five to one. The processes were used to deliver 8.5 kg of material in an overall yield of 44%.
中文翻译:

下一代口服选择性 ERα 降解剂 Camizestrant 的首次数千克合成
Camizestrant 目前正在针对 ER+ 乳腺癌的多项 3 期临床试验中进行研究。本文描述了我们为首次生产临床材料所做的努力。战略流程开发的重点是提供强大的流程和控制点,这些流程和控制点可以扩展以提供质量合适的公斤材料并满足快速的项目时间表。亮点包括优化高效的 Buchwald-Hartwig 胺化、开发非对映选择性 Pictet-Spengler 反应以及有效的分离,以及将色谱级数从 5 级显着减少到 1 级。这些工艺交付了 8.5 公斤材料,总产率为 44%。
更新日期:2024-06-07
中文翻译:

下一代口服选择性 ERα 降解剂 Camizestrant 的首次数千克合成
Camizestrant 目前正在针对 ER+ 乳腺癌的多项 3 期临床试验中进行研究。本文描述了我们为首次生产临床材料所做的努力。战略流程开发的重点是提供强大的流程和控制点,这些流程和控制点可以扩展以提供质量合适的公斤材料并满足快速的项目时间表。亮点包括优化高效的 Buchwald-Hartwig 胺化、开发非对映选择性 Pictet-Spengler 反应以及有效的分离,以及将色谱级数从 5 级显着减少到 1 级。这些工艺交付了 8.5 公斤材料,总产率为 44%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号