当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Screening metal cation additives driven by differential capacitance for Zn batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4ee01127a Zhengqiang Hu 1 , Fengling Zhang 1 , Feng Wu 1, 2, 3 , Huirong Wang 1 , Anbin Zhou 1 , Yi Chen 1 , Tianyang Xue 1 , Renjie Chen 1, 2, 3 , Li Li 1, 2, 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4ee01127a Zhengqiang Hu 1 , Fengling Zhang 1 , Feng Wu 1, 2, 3 , Huirong Wang 1 , Anbin Zhou 1 , Yi Chen 1 , Tianyang Xue 1 , Renjie Chen 1, 2, 3 , Li Li 1, 2, 3
Affiliation
|
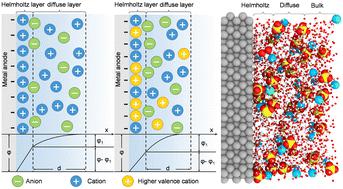
In electrochemical devices, the electric double layer (EDL) theory posits that the potential difference of the Helmholtz layer significantly influences the activation energy and reaction rate of the electrochemical reaction, which determines the uniformity of metal ion deposition. In this work, we compare the differential capacitance of metal cationic sulfate, along with factors such as cost as well as safety, and Ce(SO4)2 is selected to adjust the EDL at the Zn anode/electrolyte interface. Molecular dynamics simulations show the formation of a more compressed diffuse layer with the help of Ce4+, resulting in a decreased potential difference of the Helmholtz layer, an improved Zn2+ deposition overpotential and a reduced Zn electrode corrosion rate. In situ XRD and Raman spectroscopy reveal that Zn2+ deposition is favored along the (002) crystal plane, and hydrogen evolution is hindered. The Ce4+ modified electrolyte enables a Zn anode with high coulombic efficiency (99.6%) over 600 cycles at 10 mA cm−2. The compressed EDL extends the lifespan of the Zn–Zn cell to 2500 hours at 5 mA cm−2 and stable cycling for 300 cycles at 20 mA cm−2 and 10 mA h cm−2. Assembling Zn full cells with high-loading cathodes shows enhanced long-term cycling performance. Our findings reveal the potential of using high-valence metal cations like Ce4+ to regulate the EDL and uniform Zn2+ deposition in ZIBs, which suggests a promising direction for developing electrolytes for practical ZIBs.
中文翻译:

锌电池微分电容驱动金属阳离子添加剂的筛选
在电化学器件中,双电层(EDL)理论认为,亥姆霍兹层的电位差显着影响电化学反应的活化能和反应速率,从而决定金属离子沉积的均匀性。在这项工作中,我们比较了金属阳离子硫酸盐的微分电容,以及成本和安全性等因素,并选择Ce(SO 4 ) 2 来调整EDL在锌阳极/电解质界面处。分子动力学模拟表明,在 Ce 4+ 的帮助下形成了更压缩的扩散层,从而降低了亥姆霍兹层的电势差,提高了 Zn 2+ 沉积过电势和降低锌电极腐蚀速率。原位XRD和拉曼光谱表明,Zn 2+ 沿着(002)晶面沉积是有利的,析氢受到阻碍。 Ce 4+ 改性电解质使 Zn 阳极在 10 mA cm −2 电流下循环 600 次后具有高库仑效率 (99.6%)。压缩的 EDL 将 Zn-Zn 电池的寿命延长至 5 mA cm −2 下的 2500 小时,并在 20 mA cm −2 和 10 mA h cm −2 。将锌全电池与高负载阴极组装在一起显示出增强的长期循环性能。我们的研究结果揭示了使用 Ce 4+ 等高价金属阳离子来调节 ZIB 中 EDL 和均匀 Zn 2+ 沉积的潜力,这为开发实用 ZIB 电解质提供了一个有前景的方向。
更新日期:2024-06-07
中文翻译:

锌电池微分电容驱动金属阳离子添加剂的筛选
在电化学器件中,双电层(EDL)理论认为,亥姆霍兹层的电位差显着影响电化学反应的活化能和反应速率,从而决定金属离子沉积的均匀性。在这项工作中,我们比较了金属阳离子硫酸盐的微分电容,以及成本和安全性等因素,并选择Ce(SO 4 ) 2 来调整EDL在锌阳极/电解质界面处。分子动力学模拟表明,在 Ce 4+ 的帮助下形成了更压缩的扩散层,从而降低了亥姆霍兹层的电势差,提高了 Zn 2+ 沉积过电势和降低锌电极腐蚀速率。原位XRD和拉曼光谱表明,Zn 2+ 沿着(002)晶面沉积是有利的,析氢受到阻碍。 Ce 4+ 改性电解质使 Zn 阳极在 10 mA cm −2 电流下循环 600 次后具有高库仑效率 (99.6%)。压缩的 EDL 将 Zn-Zn 电池的寿命延长至 5 mA cm −2 下的 2500 小时,并在 20 mA cm −2 和 10 mA h cm −2 。将锌全电池与高负载阴极组装在一起显示出增强的长期循环性能。我们的研究结果揭示了使用 Ce 4+ 等高价金属阳离子来调节 ZIB 中 EDL 和均匀 Zn 2+ 沉积的潜力,这为开发实用 ZIB 电解质提供了一个有前景的方向。






































 京公网安备 11010802027423号
京公网安备 11010802027423号