当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rh(III)-catalyzed C(sp2)–H functionalization/[4+2] annulation of oxadiazolones with iodonium ylides to access diverse fused-isoquinolines and fused-pyridines
Chemical Communications ( IF 4.3 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4cc02046d Wang-Liang Chen 1 , Jia-Lin Song 1, 2 , Sheng Fang 1 , Jiong-Bang Li 1 , Shang-Shi Zhang 2 , Bing Shu 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4cc02046d Wang-Liang Chen 1 , Jia-Lin Song 1, 2 , Sheng Fang 1 , Jiong-Bang Li 1 , Shang-Shi Zhang 2 , Bing Shu 1
Affiliation
|
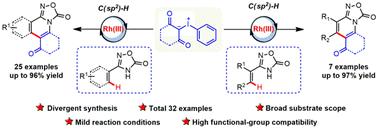
In this study, a Rh(III)-catalyzed C–H/N–H [4+2] annulation of oxadiazolones with iodonium ylides has been developed, which afforded a series of diverse fused-isoquinolines and fused-pyridines in moderate to high yields. These divergent synthesis protocols featured mild conditions, broad substrate scope, and functional-group compatibility. In addition, scale-up synthesis, related applications and preliminary mechanistic explorations were also accomplished.
中文翻译:

Rh(III)-催化恶二唑酮与碘叶立德的 C(sp2)–H 官能化/[4+2]环化,得到多种稠合异喹啉和稠合吡啶
在本研究中,开发了 Rh(III) 催化的恶二唑酮与碘叶立德的 C–H/N–H [4+2] 成环反应,在中等到高浓度下提供了一系列不同的稠合异喹啉和稠合吡啶。产量。这些不同的合成方案具有条件温和、底物范围广泛和官能团兼容性的特点。此外,还完成了放大合成、相关应用和初步机理探索。
更新日期:2024-06-07
中文翻译:

Rh(III)-催化恶二唑酮与碘叶立德的 C(sp2)–H 官能化/[4+2]环化,得到多种稠合异喹啉和稠合吡啶
在本研究中,开发了 Rh(III) 催化的恶二唑酮与碘叶立德的 C–H/N–H [4+2] 成环反应,在中等到高浓度下提供了一系列不同的稠合异喹啉和稠合吡啶。产量。这些不同的合成方案具有条件温和、底物范围广泛和官能团兼容性的特点。此外,还完成了放大合成、相关应用和初步机理探索。






































 京公网安备 11010802027423号
京公网安备 11010802027423号