当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An aryl-to-alkyl radical relay arylation reaction of a remote C(sp3)–H bond using 1,4-dicyanobenzene as an electrochemical redox-mediator
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4qo00623b Weijie Yu 1 , Hongjie Zhang 1 , Zhipeng Shen 1 , Lingyun Yang 1 , Jin Luo 2 , Wendong Li 1 , Kuang Zhao 1 , Xiaolong Li 1 , Jiale Xu 1 , Yuan Zhou 1 , Tao Wang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-07 , DOI: 10.1039/d4qo00623b Weijie Yu 1 , Hongjie Zhang 1 , Zhipeng Shen 1 , Lingyun Yang 1 , Jin Luo 2 , Wendong Li 1 , Kuang Zhao 1 , Xiaolong Li 1 , Jiale Xu 1 , Yuan Zhou 1 , Tao Wang 1
Affiliation
|
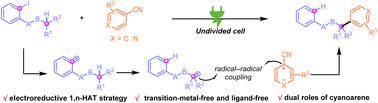
An electroreduction-enabled aryl-to-alkyl radical relay arylation reaction of the remote C(sp3)–H bond of 2-iodoalkylarenes is reported. This electrochemical strategy takes place via a 1,5-H transfer process, leading to various arylated products bearing all-carbon quaternary centers under transition-metal and ligand-free conditions. The aryl nitriles serve as both aryl radical precursors and redox mediators in this transformation.
中文翻译:

使用 1,4-二氰基苯作为电化学氧化还原介体的远程 C(sp3)-H 键的芳基到烷基自由基中继芳基化反应
报道了 2-碘代烷基芳烃的远程 C(sp 3 )–H 键的电还原芳基到烷基自由基中继芳基化反应。这种电化学策略通过 1,5-H 转移过程进行,导致在过渡金属和无配体条件下产生带有全碳四元中心的各种芳基化产物。芳基腈在此转化中既充当芳基自由基前体又充当氧化还原介体。
更新日期:2024-06-07
中文翻译:

使用 1,4-二氰基苯作为电化学氧化还原介体的远程 C(sp3)-H 键的芳基到烷基自由基中继芳基化反应
报道了 2-碘代烷基芳烃的远程 C(sp 3 )–H 键的电还原芳基到烷基自由基中继芳基化反应。这种电化学策略通过 1,5-H 转移过程进行,导致在过渡金属和无配体条件下产生带有全碳四元中心的各种芳基化产物。芳基腈在此转化中既充当芳基自由基前体又充当氧化还原介体。































 京公网安备 11010802027423号
京公网安备 11010802027423号