当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2,6-Bis(5-(tert-butyl)-1H-pyrazol-3-yl)pyridine: Effects of the Peripheral Aliphatic Side Chain on the Coordination of Actinides(III) and Lanthanides(III)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.inorgchem.4c00396 Jonas Stracke 1, 2 , Patrik Weßling 2 , Thomas Sittel 1 , Christian Adam 1 , Frank Rominger 3 , Andreas Geist 1 , Petra J Panak 1, 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.inorgchem.4c00396 Jonas Stracke 1, 2 , Patrik Weßling 2 , Thomas Sittel 1 , Christian Adam 1 , Frank Rominger 3 , Andreas Geist 1 , Petra J Panak 1, 2
Affiliation
|
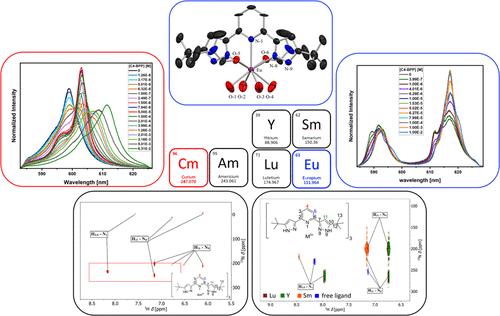
To improve our understanding of the interaction mechanism in trivalent lanthanide and actinide complexes, studies with structurally different hard and soft donor ligands are of great interest. For that reason, the coordination chemistry of An(III) and Ln(III) with 2,6-bis(5-(tert-butyl)-1H-pyrazol-3-yl)pyridine (C4-BPP) has been explored. Time-resolved laser fluorescence spectroscopy (TRLFS) studies have revealed the formation of [Cm(C4-BPP)n]3+ (n = 1–3) (log β1′ = 7.2 ± 0.4, log β2′ = 10.1 ± 0.5, and log β3′ = 11.8 ± 0.6) and [Eu(C4-BPP)m]3+ (m = 1–2) (log β1′ = 4.9 ± 0.2 and log β2′ = 8.0 ± 0.4). The absence of the [Eu(C4-BPP)3]3+ complex shows a more favorable complexation of Cm(III) over that of Eu(III). Additionally, complementary NMR measurements have been conducted to examine the M(III)–N bond in Ln(III) and Am(III) C4-BPP complexes. 15N NMR data have revealed notable differences in the chemical shifts of the coordinating nitrogen atoms between the Am(III) and Ln(III) complexes. In the Am(III) complex, the coordinating nitrogen atoms have shown a shift by 260 ppm, indicating a higher fraction of covalent bonding in the Am(III)–N bond compared with the Ln(III)–N bond. This observation aligns excellently with the differences in the stability constants obtained from TRLFS studies.
中文翻译:

2,6-双(5-(叔丁基)-1H-吡唑-3-基)吡啶:外围脂肪族侧链对锕系(III)和镧系(III)配位的影响
为了提高我们对三价镧系元素和锕系元素配合物相互作用机制的理解,对结构不同的硬和软供体配体的研究非常有意义。因此,我们探索了 An(III) 和 Ln(III) 与 2,6-双(5-(叔丁基)-1 H-吡唑-3-基)吡啶 (C4-BPP) 的配位化学。时间分辨激光荧光光谱 (TRLFS) 研究揭示了 [Cm(C4-BPP) n ] 3+ ( n = 1–3) (log β 1 ′ = 7.2 ± 0.4, log β 2 ′ = 10.1 ± 0.5,log β 3 ′ = 11.8 ± 0.6) 和 [Eu(C4-BPP) m ] 3+ ( m = 1–2) (log β 1 ′ = 4.9 ± 0.2 和 log β 2 ′ = 8.0 ± 0.4) 。 [Eu(C4-BPP) 3 ] 3+络合物的不存在表明Cm(III)比Eu(III)更有利的络合。此外,还进行了补充 NMR 测量来检查 Ln(III) 和 Am(III) C4-BPP 复合物中的 M(III)-N 键。 15 N NMR 数据揭示了 Am(III) 和 Ln(III) 配合物之间配位氮原子的化学位移存在显着差异。在 Am(III) 配合物中,配位氮原子发生了 260 ppm 的偏移,表明与 Ln(III)-N 键相比,Am(III)-N 键中的共价键比例更高。这一观察结果与 TRLFS 研究中获得的稳定性常数的差异非常吻合。
更新日期:2024-06-06
中文翻译:

2,6-双(5-(叔丁基)-1H-吡唑-3-基)吡啶:外围脂肪族侧链对锕系(III)和镧系(III)配位的影响
为了提高我们对三价镧系元素和锕系元素配合物相互作用机制的理解,对结构不同的硬和软供体配体的研究非常有意义。因此,我们探索了 An(III) 和 Ln(III) 与 2,6-双(5-(叔丁基)-1 H-吡唑-3-基)吡啶 (C4-BPP) 的配位化学。时间分辨激光荧光光谱 (TRLFS) 研究揭示了 [Cm(C4-BPP) n ] 3+ ( n = 1–3) (log β 1 ′ = 7.2 ± 0.4, log β 2 ′ = 10.1 ± 0.5,log β 3 ′ = 11.8 ± 0.6) 和 [Eu(C4-BPP) m ] 3+ ( m = 1–2) (log β 1 ′ = 4.9 ± 0.2 和 log β 2 ′ = 8.0 ± 0.4) 。 [Eu(C4-BPP) 3 ] 3+络合物的不存在表明Cm(III)比Eu(III)更有利的络合。此外,还进行了补充 NMR 测量来检查 Ln(III) 和 Am(III) C4-BPP 复合物中的 M(III)-N 键。 15 N NMR 数据揭示了 Am(III) 和 Ln(III) 配合物之间配位氮原子的化学位移存在显着差异。在 Am(III) 配合物中,配位氮原子发生了 260 ppm 的偏移,表明与 Ln(III)-N 键相比,Am(III)-N 键中的共价键比例更高。这一观察结果与 TRLFS 研究中获得的稳定性常数的差异非常吻合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号