当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid End-Game Process Development and First GMP Production of MK-7845: An Experimental Antiviral Treatment for COVID-19
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.oprd.4c00015 Nicholas R. Deprez 1 , Jonathan M. E. Hughes 1 , Shorouk O. Badir 1 , Stasik Popov 1 , Teresa Andreani 1 , Rachel S. Bade 1 , Clara Hartmanshenn 1 , Thomas Tai-min Kwok 1 , Donald R. Gauthier 1 , Nastaran Salehi Marzijarani 1 , Zeinab Sakhaei 1 , Riki Drout 1 , Steve Castro 1 , David J. Schenk 1 , Charles Wolstenholme 1 , Nilusha Padivitage 1 , Cody Welch 1 , Jason R. Kowalski 1 , Brittany Kassim 1 , Yong Liu 1 , Ryan D. Cohen 1 , Alex M. Confer 1 , Guilherme Dal Poggetto 1 , Andrew P. J. Brunskill 1 , Feng Peng 1 , Ji Qi 1 , Jing Xu 1 , Mingxiang Lin 1 , Jamie M. McCabe Dunn 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.oprd.4c00015 Nicholas R. Deprez 1 , Jonathan M. E. Hughes 1 , Shorouk O. Badir 1 , Stasik Popov 1 , Teresa Andreani 1 , Rachel S. Bade 1 , Clara Hartmanshenn 1 , Thomas Tai-min Kwok 1 , Donald R. Gauthier 1 , Nastaran Salehi Marzijarani 1 , Zeinab Sakhaei 1 , Riki Drout 1 , Steve Castro 1 , David J. Schenk 1 , Charles Wolstenholme 1 , Nilusha Padivitage 1 , Cody Welch 1 , Jason R. Kowalski 1 , Brittany Kassim 1 , Yong Liu 1 , Ryan D. Cohen 1 , Alex M. Confer 1 , Guilherme Dal Poggetto 1 , Andrew P. J. Brunskill 1 , Feng Peng 1 , Ji Qi 1 , Jing Xu 1 , Mingxiang Lin 1 , Jamie M. McCabe Dunn 1
Affiliation
|
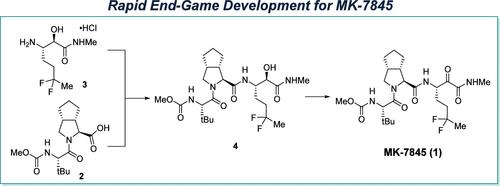
We describe the rapid end-game process development for the first good manufacturing process (GMP) delivery of the 3C-like protease inhibitor MK-7845 (1), an experimental treatment for SARS-CoV-2. Three operations, including an amide-coupling, oxidation, and crystallization, were rapidly developed and implemented on a kilogram scale to enable critical safety studies and phase 1 clinical trials to move forward on a highly accelerated timeline. Key to the success of this undertaking was our focus on purging key impurities formed in the amide-coupling step, identifying a safe and scalable TEMPO/NaOCl oxidation to access 1, and developing an active pharmacutical ingredient (API) crystallization that addressed challenges associated with gumming, oiling, and agglomeration. Notably, this delivery was completed within an approximately six-week time frame, and challenges associated with this highly accelerated delivery are also discussed.
中文翻译:

MK-7845 的快速最终工艺开发和首次 GMP 生产:针对 COVID-19 的实验性抗病毒治疗
我们描述了 3C 样蛋白酶抑制剂 MK-7845(一种针对 SARS-CoV-2 的实验性治疗方法)的首次良好生产工艺 (GMP) 交付的快速最终流程开发 (1)。包括酰胺偶联、氧化和结晶在内的三项操作得到了快速开发并在公斤级上实施,从而使关键的安全性研究和一期临床试验能够在高度加速的时间内取得进展。这项工作成功的关键是我们专注于清除酰胺偶联步骤中形成的关键杂质,确定安全且可扩展的 TEMPO/NaOCl 氧化方法以获取 1,并开发一种活性药物成分 (API) 结晶来解决与涂胶、涂油和结块。值得注意的是,这次交付在大约六周的时间内完成,并且还讨论了与这种高度加速的交付相关的挑战。
更新日期:2024-06-06
中文翻译:

MK-7845 的快速最终工艺开发和首次 GMP 生产:针对 COVID-19 的实验性抗病毒治疗
我们描述了 3C 样蛋白酶抑制剂 MK-7845(一种针对 SARS-CoV-2 的实验性治疗方法)的首次良好生产工艺 (GMP) 交付的快速最终流程开发 (1)。包括酰胺偶联、氧化和结晶在内的三项操作得到了快速开发并在公斤级上实施,从而使关键的安全性研究和一期临床试验能够在高度加速的时间内取得进展。这项工作成功的关键是我们专注于清除酰胺偶联步骤中形成的关键杂质,确定安全且可扩展的 TEMPO/NaOCl 氧化方法以获取 1,并开发一种活性药物成分 (API) 结晶来解决与涂胶、涂油和结块。值得注意的是,这次交付在大约六周的时间内完成,并且还讨论了与这种高度加速的交付相关的挑战。






































 京公网安备 11010802027423号
京公网安备 11010802027423号