当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process Development of a Tricyclic Diazepine-Based IDH1 Mutant Inhibitor
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.oprd.4c00171 Matthew L. Maddess 1 , Ed Cleator 2 , Mariko Morimoto 1 , Adrian Goodyear 2 , Alejandro Dieguez-Vazquez 2 , Andrew Gibb 2 , Andy Kirtley 2 , Melodie Christensen 3 , Chaohui Song 4 , Feng Peng 3 , Mahbub Alam 2 , Stephen P. Keen 2 , Steven F. Oliver 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-06-06 , DOI: 10.1021/acs.oprd.4c00171 Matthew L. Maddess 1 , Ed Cleator 2 , Mariko Morimoto 1 , Adrian Goodyear 2 , Alejandro Dieguez-Vazquez 2 , Andrew Gibb 2 , Andy Kirtley 2 , Melodie Christensen 3 , Chaohui Song 4 , Feng Peng 3 , Mahbub Alam 2 , Stephen P. Keen 2 , Steven F. Oliver 2
Affiliation
|
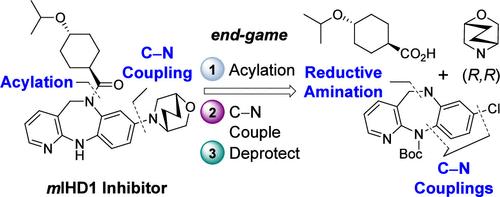
Process development to improve synthetic access to a potent, selective, and brain-penetrant tricyclic diazepine clinical candidate that inhibits mutant IDH1 is described. A variety of disconnections were evaluated to determine the preferred sequence of fragment coupling. The optimized route involves a metal-catalyzed C–N coupling/reductive cascade to form the central diazepine core, improved entries to both the zigzag morpholine and cyclohexyl acid peripheral pieces, and an efficient end-game sequence of acylation, C–N coupling, and deprotection. In addition, a dynamic acylation process that enables selective acylation at N6 of an unprotected diazepine core is described.
中文翻译:

基于三环二氮杂卓的 IDH1 突变抑制剂的工艺开发
描述了改进合成获得有效、选择性和脑渗透性三环二氮杂卓临床候选药物的工艺开发,该候选药物可抑制突变 IDH1。评估了各种断开以确定片段偶联的首选顺序。优化的路线涉及金属催化的 C-N 偶联/还原级联以形成中央二氮杂卓核心,改进了锯齿形吗啉和环己酸外围片段的进入,以及有效的酰化、C-N 偶联、和去保护。此外,还描述了一种动态酰化过程,该过程能够在未受保护的二氮杂卓核心的 N6 处进行选择性酰化。
更新日期:2024-06-06
中文翻译:

基于三环二氮杂卓的 IDH1 突变抑制剂的工艺开发
描述了改进合成获得有效、选择性和脑渗透性三环二氮杂卓临床候选药物的工艺开发,该候选药物可抑制突变 IDH1。评估了各种断开以确定片段偶联的首选顺序。优化的路线涉及金属催化的 C-N 偶联/还原级联以形成中央二氮杂卓核心,改进了锯齿形吗啉和环己酸外围片段的进入,以及有效的酰化、C-N 偶联、和去保护。此外,还描述了一种动态酰化过程,该过程能够在未受保护的二氮杂卓核心的 N6 处进行选择性酰化。






































 京公网安备 11010802027423号
京公网安备 11010802027423号