当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Spirophosphoric-Acid-Catalyzed Divergent Vinylogous Mannich and aza-Friedel–Crafts Reactions of 2-Methoxyfuran
Organic Letters ( IF 4.9 ) Pub Date : 2024-06-05 , DOI: 10.1021/acs.orglett.4c01458 Yi-Han Chao, Paru Jamwal, Gunda Ananda Rao, Ramani Gurubrahamam, Kwunmin Chen
Organic Letters ( IF 4.9 ) Pub Date : 2024-06-05 , DOI: 10.1021/acs.orglett.4c01458 Yi-Han Chao, Paru Jamwal, Gunda Ananda Rao, Ramani Gurubrahamam, Kwunmin Chen
|
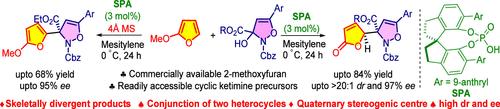
The first enantioselective vinylogous Mannich reaction is developed using 2-methoxyfuran under chiral spirophosphoric acid catalysis. The strategy involves 4-isoxazoline derivatives as cyclic ketimine surrogates and provides γ-butenolide scaffolds (up to 97% ee and >20:1 dr). The mechanistic investigations suggest that an in situ generated water molecule plays a crucial role in delivering γ-butenolide, while the use of molecular sieves delivers aza-Friedel–Crafts products. The synthetic utility of γ-butenolide is shown toward obtaining piperidone skeleton via a lactone-lactam rearrangement.
中文翻译:

手性螺磷酸催化 2-甲氧基呋喃的发散乙烯基曼尼希和氮杂弗里德尔-克来福特反应
第一个对映选择性插烯曼尼希反应是在手性螺磷酸催化下使用 2-甲氧基呋喃开发的。该策略涉及 4-异恶唑啉衍生物作为环状酮亚胺替代物,并提供 γ-丁烯内酯支架(高达 97% ee 和 >20:1 dr)。机理研究表明,原位生成的水分子在输送γ-丁烯内酯方面发挥着至关重要的作用,而使用分子筛则可输送氮杂-弗里德尔-克拉夫茨产品。 γ-丁烯内酯的合成用途被证明可通过内酯-内酰胺重排获得哌啶酮骨架。
更新日期:2024-06-05
中文翻译:

手性螺磷酸催化 2-甲氧基呋喃的发散乙烯基曼尼希和氮杂弗里德尔-克来福特反应
第一个对映选择性插烯曼尼希反应是在手性螺磷酸催化下使用 2-甲氧基呋喃开发的。该策略涉及 4-异恶唑啉衍生物作为环状酮亚胺替代物,并提供 γ-丁烯内酯支架(高达 97% ee 和 >20:1 dr)。机理研究表明,原位生成的水分子在输送γ-丁烯内酯方面发挥着至关重要的作用,而使用分子筛则可输送氮杂-弗里德尔-克拉夫茨产品。 γ-丁烯内酯的合成用途被证明可通过内酯-内酰胺重排获得哌啶酮骨架。







































 京公网安备 11010802027423号
京公网安备 11010802027423号