Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Capturing totipotency in human cells through spliceosomal repression
Cell ( IF 45.5 ) Pub Date : 2024-06-05 , DOI: 10.1016/j.cell.2024.05.010 Shiyu Li 1 , Min Yang 1 , Hui Shen 1 , Li Ding 1 , Xuehui Lyu 1 , Kexin Lin 1 , Jennie Ong 2 , Peng Du 3
Cell ( IF 45.5 ) Pub Date : 2024-06-05 , DOI: 10.1016/j.cell.2024.05.010 Shiyu Li 1 , Min Yang 1 , Hui Shen 1 , Li Ding 1 , Xuehui Lyu 1 , Kexin Lin 1 , Jennie Ong 2 , Peng Du 3
Affiliation
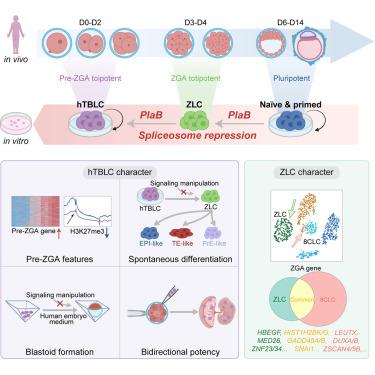
|
The cleavage of zygotes generates totipotent blastomeres. In human 8-cell blastomeres, zygotic genome activation (ZGA) occurs to initiate the ontogenesis program. However, capturing and maintaining totipotency in human cells pose significant challenges. Here, we realize culturing human totipotent blastomere-like cells (hTBLCs). We find that splicing inhibition can transiently reprogram human pluripotent stem cells into ZGA-like cells (ZLCs), which subsequently transition into stable hTBLCs after long-term passaging. Distinct from reported 8-cell-like cells (8CLCs), both ZLCs and hTBLCs widely silence pluripotent genes. Interestingly, ZLCs activate a particular group of ZGA-specific genes, and hTBLCs are enriched with pre-ZGA-specific genes. During spontaneous differentiation, hTBLCs re-enter the intermediate ZLC stage and further generate epiblast (EPI)-, primitive endoderm (PrE)-, and trophectoderm (TE)-like lineages, effectively recapitulating human pre-implantation development. Possessing both embryonic and extraembryonic developmental potency, hTBLCs can autonomously generate blastocyst-like structures without external cell signaling. In summary, our study provides key criteria and insights into human cell totipotency.
中文翻译:

通过剪接体抑制捕获人类细胞的全能性
受精卵的分裂产生全能卵裂球。在人类 8 细胞卵裂球中,合子基因组激活 (ZGA) 发生以启动个体发生程序。然而,捕获和维持人类细胞的全能性提出了重大挑战。在这里,我们实现了人全能卵裂球样细胞(hTBLC)的培养。我们发现剪接抑制可以将人类多能干细胞瞬时重编程为ZGA样细胞(ZLC),随后在长期传代后转变为稳定的hTBLC。与报道的 8 细胞样细胞 (8CLC) 不同,ZLC 和 hTBLC 都广泛沉默多能基因。有趣的是,ZLC 激活一组特定的 ZGA 特异性基因,而 hTBLC 富含前 ZGA 特异性基因。在自发分化过程中,hTBLC 重新进入中间 ZLC 阶段,并进一步生成外胚层 (EPI)、原始内胚层 (PrE) 和滋养外胚层 (TE) 样谱系,有效地再现了人类植入前发育。 hTBLC 具有胚胎和胚胎外发育能力,无需外部细胞信号传导即可自主生成囊胚样结构。总之,我们的研究提供了人类细胞全能性的关键标准和见解。
更新日期:2024-06-05
中文翻译:

通过剪接体抑制捕获人类细胞的全能性
受精卵的分裂产生全能卵裂球。在人类 8 细胞卵裂球中,合子基因组激活 (ZGA) 发生以启动个体发生程序。然而,捕获和维持人类细胞的全能性提出了重大挑战。在这里,我们实现了人全能卵裂球样细胞(hTBLC)的培养。我们发现剪接抑制可以将人类多能干细胞瞬时重编程为ZGA样细胞(ZLC),随后在长期传代后转变为稳定的hTBLC。与报道的 8 细胞样细胞 (8CLC) 不同,ZLC 和 hTBLC 都广泛沉默多能基因。有趣的是,ZLC 激活一组特定的 ZGA 特异性基因,而 hTBLC 富含前 ZGA 特异性基因。在自发分化过程中,hTBLC 重新进入中间 ZLC 阶段,并进一步生成外胚层 (EPI)、原始内胚层 (PrE) 和滋养外胚层 (TE) 样谱系,有效地再现了人类植入前发育。 hTBLC 具有胚胎和胚胎外发育能力,无需外部细胞信号传导即可自主生成囊胚样结构。总之,我们的研究提供了人类细胞全能性的关键标准和见解。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号