当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective C2 and C3 phosphorylmethylation of indoles with a phosphorylmethyl dibenzothiophenium reagent
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-05 , DOI: 10.1039/d4qo00611a Xiaomin Shi 1, 2 , Hongmei Qu 1 , Yaxing Wu 2 , Fei Wang 2 , Chao Chen 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-05 , DOI: 10.1039/d4qo00611a Xiaomin Shi 1, 2 , Hongmei Qu 1 , Yaxing Wu 2 , Fei Wang 2 , Chao Chen 2
Affiliation
|
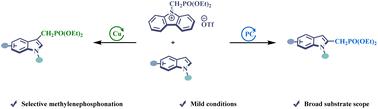
A novel electrophilic phosphorylmethylating reagent, S-([diethoxyphosphoryl]methyl) dibenzothiophenium salt, has been successfully synthesized with readily available starting materials. This reagent is air- and moisture-tolerant and bench-stable. Interestingly, the reagent exhibited excellent selectivity for the formation of indole C(sp2)–CH2PO(OEt)2 bonds at position C2 with a photocatalyst and at position C3 with a copper catalyst. Moreover, the resulting compounds with CH2PO(OEt)2 could be easily converted into free phosphinic acid, providing a new pathway for preparing complex acid molecules.
中文翻译:

使用磷酰甲基二苯并噻吩鎓试剂对吲哚进行选择性 C2 和 C3 磷酰甲基化
一种新型亲电磷酰甲基化试剂,S-([二乙氧基磷酰基]甲基)二苯并噻吩鎓盐,已用易于获得的起始原料成功合成。该试剂具有耐空气性和耐湿性,并且在工作台上稳定。有趣的是,该试剂在光催化剂作用下,在 C2 位形成吲哚 C(sp 2 )–CH 2 PO(OEt) 2 键时表现出优异的选择性并在位置C3用铜催化剂。此外,所得的CH 2 PO(OEt) 2 化合物可以很容易地转化为游离次膦酸,为制备复杂酸分子提供了新的途径。
更新日期:2024-06-05
中文翻译:

使用磷酰甲基二苯并噻吩鎓试剂对吲哚进行选择性 C2 和 C3 磷酰甲基化
一种新型亲电磷酰甲基化试剂,S-([二乙氧基磷酰基]甲基)二苯并噻吩鎓盐,已用易于获得的起始原料成功合成。该试剂具有耐空气性和耐湿性,并且在工作台上稳定。有趣的是,该试剂在光催化剂作用下,在 C2 位形成吲哚 C(sp 2 )–CH 2 PO(OEt) 2 键时表现出优异的选择性并在位置C3用铜催化剂。此外,所得的CH 2 PO(OEt) 2 化合物可以很容易地转化为游离次膦酸,为制备复杂酸分子提供了新的途径。






































 京公网安备 11010802027423号
京公网安备 11010802027423号