Chemical Research in Chinese Universities ( IF 3.1 ) Pub Date : 2024-06-04 , DOI: 10.1007/s40242-024-4075-8 Junjie Zhang , Lunan Lv , Haoran Zhu , Ying Zhang , Xiaodi Xu , Lanxin Long , Wei Fu
|
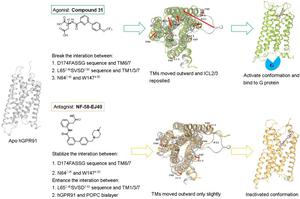
G protein-coupled receptor 91 (GPR91) has garnered widespread attention as a prospective therapeutic target for metabolic diseases. However, no structural data for human GPR91 (hGPR91) and detailed molecular mechanism of action (MOA) of GPR91 have been reported, with reported compounds targeting GPR91 limited. In this study, hGPR91 structures were constructed through homology modeling. High-affinity agonist compound 31 and antagonist NF-58-EJ40 were selected for investigation. By molecular dynamics (MD) simulations, we have elucidated MOA of GPR91 agonists and antagonists for the first time. We identified the crucial role of the D174FASSG sequence, the L652.46SVSD2.50 sequence and the N642.45-W1474.50 in maintaining GPR91’s inactive state conformation. Agonist binding disrupted constraints mediated by the aforementioned sequence, which led to significant outward movements of transmembrane helixes (TMs) and repositioning of intracellular loop2 (ICL2) and ICL3, thereby forming an expanded cavity for G proteins binding. Furthermore, the pivotal role of the dicarboxylic acid structure of agonists in initiating signal transduction was confirmed. In contrast, antagonist binding stabilized these conformational constraints, resulting in relatively minor movements of TMs that were insufficient to generate a binding cavity large enough to accommodate the G protein. Clarifying MOA of GPR91 agonists and antagonists is crucial for guiding the design of relevant drugs.
中文翻译:

GPR91 激动剂和拮抗剂的分子作用机制:分子动力学模拟的见解
G蛋白偶联受体91(GPR91)作为代谢性疾病的潜在治疗靶点而受到广泛关注。然而,尚未报道人GPR91(hGPR91)的结构数据和GPR91详细的分子作用机制(MOA),报道的针对GPR91的化合物有限。在本研究中,通过同源建模构建了hGPR91结构。选择高亲和力激动剂化合物31和拮抗剂NF-58-EJ40进行研究。通过分子动力学(MD)模拟,我们首次阐明了GPR91激动剂和拮抗剂的MOA。我们确定了 D174FASSG 序列、L65 2.46 SVSD 2.50 序列和 N64 2.45 -W147 4.50 在维持 GPR91 中的关键作用非活性状态构象。激动剂结合破坏了上述序列介导的限制,导致跨膜螺旋 (TM) 显着向外移动以及细胞内环 2 (ICL2) 和 ICL3 的重新定位,从而形成用于 G 蛋白结合的扩大空腔。此外,还证实了激动剂的二羧酸结构在启动信号转导中的关键作用。相反,拮抗剂结合稳定了这些构象限制,导致 TM 的运动相对较小,不足以产生足够大的结合腔来容纳 G 蛋白。明确GPR91激动剂和拮抗剂的作用机制对于指导相关药物的设计至关重要。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号