Cell ( IF 45.5 ) Pub Date : 2024-06-04 , DOI: 10.1016/j.cell.2024.05.002 Sarah W. Cai , Hiroyuki Takai , Arthur J. Zaug , Teague C. Dilgen , Thomas R. Cech , Thomas Walz , Titia de Lange
|
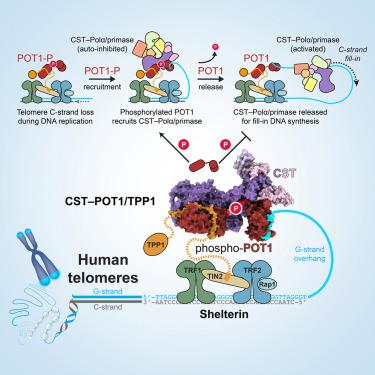
Telomere maintenance requires the extension of the G-rich telomeric repeat strand by telomerase and the fill-in synthesis of the C-rich strand by Polα/primase. At telomeres, Polα/primase is bound to Ctc1/Stn1/Ten1 (CST), a single-stranded DNA-binding complex. Like mutations in telomerase, mutations affecting CST-Polα/primase result in pathological telomere shortening and cause a telomere biology disorder, Coats plus (CP). We determined cryogenic electron microscopy structures of human CST bound to the shelterin heterodimer POT1/TPP1 that reveal how CST is recruited to telomeres by POT1. Our findings suggest that POT1 hinge phosphorylation is required for CST recruitment, and the complex is formed through conserved interactions involving several residues mutated in CP. Our structural and biochemical data suggest that phosphorylated POT1 holds CST-Polα/primase in an inactive, autoinhibited state until telomerase has extended the telomere ends. We propose that dephosphorylation of POT1 releases CST-Polα/primase into an active state that completes telomere replication through fill-in synthesis.
中文翻译:

POT1 在人类端粒处招募并调节 CST-Polα/引物酶
端粒的维持需要通过端粒酶延伸富含G的端粒重复链,并通过Polα/引物酶填充合成富含C的端粒重复链。在端粒处,Polα/引物酶与单链 DNA 结合复合物 Ctc1/Stn1/Ten1 (CST) 结合。与端粒酶突变一样,影响 CST-Polα/引物酶的突变会导致病理性端粒缩短并导致端粒生物学紊乱,Coats plus (CP)。我们确定了与庇护蛋白异二聚体 POT1/TPP1 结合的人 CST 的低温电子显微镜结构,揭示了 CST 如何被 POT1 募集到端粒上。我们的研究结果表明,POT1 铰链磷酸化是 CST 募集所必需的,并且该复合物是通过涉及 CP 中突变的几个残基的保守相互作用形成的。我们的结构和生化数据表明,磷酸化的 POT1 使 CST-Polα/引物酶保持在非活性、自抑制状态,直到端粒酶延长端粒末端。我们建议 POT1 的去磷酸化将 CST-Polα/引物酶释放到活性状态,通过填充合成完成端粒复制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号