当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Fe2(SO4)3-Fe2O3 interface induced by OCFGs electrostatic anchoring on AC surface: High efficiency NH3-SCR performance at low temperatures
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-05-31 , DOI: 10.1016/j.seppur.2024.128210 Pan Li , Yan Huang , Simi Li , Mouli Liu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-05-31 , DOI: 10.1016/j.seppur.2024.128210 Pan Li , Yan Huang , Simi Li , Mouli Liu
|
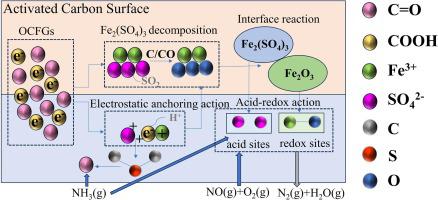
Low-temperature activity and SO2 poisoning were the key factors limiting the application of NH3 -SCR catalysts. In this study, Fe2 (SO4 )3 /AC and Fe2 (SO4 )3 /OAC catalysts were prepared by oxidation function modification and incipient wetness impregnation. In the temperature range of 100–250 ℃, the catalytic performance and SO2 resistance of Fe2 (SO4 )3 /AC and Fe2 (SO4 )3 /OAC catalysts were investigated, the denitrification efficiency increased from 28 % (Fe2 (SO4 )3 /AC) to 80 % at 100 °C and reached 100 % at 170 °C, and 100 ppm SO2 was introduced at 250 ℃ for 24 h, the denitrification efficiency remains 100 % unchanged. The mechanism of activity enhancement was further explored by BET, XRD, ICP, TG, XPS, FT-IR, H2 -TPR and NH3 -TPD. The results showed that (NH4 )2 S2 O8 modification enhanced the surface acidity, and the increase of surface oxygen-containing functional groups improved the redox performance. At the same time, due to electrostatic anchoring effects of oxygen-containing functional groups, Fe3+ and SO4 2- were adsorbed at different sites and promoted the binding of S to the carbon skeleton to form −C-S-C-. Thus, the interaction between OAC and Fe2 (SO4 )3 caused part of Fe2 (SO4 )3 to decompose into Fe2 O3 , and the formation of Fe2 (SO4 )3 -Fe2 O3 interface further enhanced the acidity and redox properties. More importantly, the decomposition temperature of NH4 HSO4 was lower and the decomposition was more thorough on the Fe2 (SO4 )3 /OAC than that of Fe2 (SO4 )3 /AC. Finally, the possible mechanism of (NH4 )2 S2 O8 -modified Fe2 (SO4 )3 /OAC catalyst to improve NH3 -SCR performance was proposed, which is of great significance for the development of NH3 -SCR catalyst with sulfur resistance at low temperatures.
中文翻译:

OCFG 静电锚定在 AC 表面诱导 Fe2(SO4)3-Fe2O3 界面的形成:低温下高效 NH3-SCR 性能
低温活性和SO2中毒是限制NH3-SCR催化剂应用的关键因素。本研究通过氧化功能改性和初湿浸渍制备了Fe2(SO4)3/AC和Fe2(SO4)3/OAC催化剂。在100~250 ℃温度范围内,考察了Fe2(SO4)3/AC和Fe2(SO4)3/OAC催化剂的催化性能和抗SO2性能,脱硝效率从28%提高(Fe2(SO4)3/ AC)在100℃时达到80%,在170℃时达到100%,并在250℃下通入100 ppm SO2 24 h,反硝化效率保持100%不变。通过BET、XRD、ICP、TG、XPS、FT-IR、H2-TPR和NH3-TPD进一步探讨了活性增强的机制。结果表明,(NH4)2S2O8改性增强了表面酸性,表面含氧官能团的增加提高了氧化还原性能。同时,由于含氧官能团的静电锚定效应,Fe3+和SO42-被吸附在不同位点,促进S与碳骨架结合形成-CSC-。因此,OAC与Fe2(SO4)3之间的相互作用导致部分Fe2(SO4)3分解为Fe2O3,并且Fe2(SO4)3-Fe2O3界面的形成进一步增强了酸性和氧化还原性能。更重要的是,与Fe2(SO4)3/AC相比,NH4HSO4在Fe2(SO4)3/OAC上的分解温度更低,分解更彻底。最后提出了(NH4)2S2O8改性Fe2(SO4)3/OAC催化剂改善NH3-SCR性能的可能机制,这对于开发低温抗硫NH3-SCR催化剂具有重要意义。
更新日期:2024-05-31
中文翻译:

OCFG 静电锚定在 AC 表面诱导 Fe2(SO4)3-Fe2O3 界面的形成:低温下高效 NH3-SCR 性能
低温活性和SO2中毒是限制NH3-SCR催化剂应用的关键因素。本研究通过氧化功能改性和初湿浸渍制备了Fe2(SO4)3/AC和Fe2(SO4)3/OAC催化剂。在100~250 ℃温度范围内,考察了Fe2(SO4)3/AC和Fe2(SO4)3/OAC催化剂的催化性能和抗SO2性能,脱硝效率从28%提高(Fe2(SO4)3/ AC)在100℃时达到80%,在170℃时达到100%,并在250℃下通入100 ppm SO2 24 h,反硝化效率保持100%不变。通过BET、XRD、ICP、TG、XPS、FT-IR、H2-TPR和NH3-TPD进一步探讨了活性增强的机制。结果表明,(NH4)2S2O8改性增强了表面酸性,表面含氧官能团的增加提高了氧化还原性能。同时,由于含氧官能团的静电锚定效应,Fe3+和SO42-被吸附在不同位点,促进S与碳骨架结合形成-CSC-。因此,OAC与Fe2(SO4)3之间的相互作用导致部分Fe2(SO4)3分解为Fe2O3,并且Fe2(SO4)3-Fe2O3界面的形成进一步增强了酸性和氧化还原性能。更重要的是,与Fe2(SO4)3/AC相比,NH4HSO4在Fe2(SO4)3/OAC上的分解温度更低,分解更彻底。最后提出了(NH4)2S2O8改性Fe2(SO4)3/OAC催化剂改善NH3-SCR性能的可能机制,这对于开发低温抗硫NH3-SCR催化剂具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号