当前位置:
X-MOL 学术
›
Nano Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mastering cathode and anode electrolyte interphases for vanadium-based zinc batteries via solvation structure control
Nano Energy ( IF 16.8 ) Pub Date : 2024-05-31 , DOI: 10.1016/j.nanoen.2024.109822
Ziwei Zhao , Pengcheng Li , Ziwei Chai , Hao Zhang , Ge Li
Nano Energy ( IF 16.8 ) Pub Date : 2024-05-31 , DOI: 10.1016/j.nanoen.2024.109822
Ziwei Zhao , Pengcheng Li , Ziwei Chai , Hao Zhang , Ge Li
|
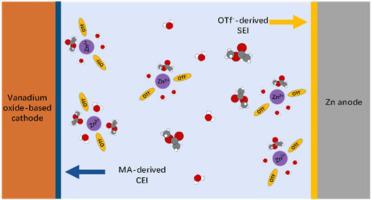
Metallic zinc-based anodes often encounter challenges such as dendrite growth, side reactions, and by-product generation, leading to diminished reversibility. This study introduces a novel approach by introducing methyl acetate as a cosolvent into an aqueous electrolyte based on Zn(OTf)2 through a salting-in effect. Regulating the solvation sheath structure of Zn2+ enables the formation of cathode electrolyte interphase and anode electrolyte interphase, enhancing the stability of the vanadium-base cathode and zinc metal anode, respectively. The incorporation of this cosolvent allows for prolonged operation of Zn//Cu half-cells for over 1500 cycles with the average columbic efficiency of 99.82 % under a current density of 1 mA cm−2 . When zinc anodes are paired with NaV3 O8 ·1.5 H2 O cathodes, a remarkable 80 % of the capacity can be maintained after 1500 cycles under a current density of 1 A/g. Furthermore, even under conditions of a 6 mg cm−2 mass loading and a negative to positive ratio of 3, the full battery demonstrates 77 % capacity retention after 300 cycles. This dual-interphase strategy provides valuable insights into advancing the stability of vanadium-based zinc metal batteries.
中文翻译:

通过溶剂化结构控制掌握钒基锌电池的阴极和阳极电解质界面
金属锌基阳极经常遇到枝晶生长、副反应和副产物生成等挑战,导致可逆性降低。本研究引入了一种新方法,通过盐析效应将乙酸甲酯作为共溶剂引入到基于 Zn(OTf)2 的水性电解质中。调节Zn2+的溶剂化鞘结构能够形成阴极电解质界面和阳极电解质界面,分别增强钒基阴极和锌金属阳极的稳定性。这种共溶剂的加入使得 Zn//Cu 半电池能够延长运行超过 1500 个周期,在 1 mA cm−2 的电流密度下平均库仑效率为 99.82%。当锌阳极与NaV3O8·1.5 H2O阴极配对时,在1 A/g的电流密度下循环1500次后仍能保持80%的容量。此外,即使在质量负载为6 mg cm−2、正负极比为3的条件下,全电池在300次循环后仍表现出77%的容量保持率。这种双界面策略为提高钒基锌金属电池的稳定性提供了宝贵的见解。
更新日期:2024-05-31
中文翻译:

通过溶剂化结构控制掌握钒基锌电池的阴极和阳极电解质界面
金属锌基阳极经常遇到枝晶生长、副反应和副产物生成等挑战,导致可逆性降低。本研究引入了一种新方法,通过盐析效应将乙酸甲酯作为共溶剂引入到基于 Zn(OTf)2 的水性电解质中。调节Zn2+的溶剂化鞘结构能够形成阴极电解质界面和阳极电解质界面,分别增强钒基阴极和锌金属阳极的稳定性。这种共溶剂的加入使得 Zn//Cu 半电池能够延长运行超过 1500 个周期,在 1 mA cm−2 的电流密度下平均库仑效率为 99.82%。当锌阳极与NaV3O8·1.5 H2O阴极配对时,在1 A/g的电流密度下循环1500次后仍能保持80%的容量。此外,即使在质量负载为6 mg cm−2、正负极比为3的条件下,全电池在300次循环后仍表现出77%的容量保持率。这种双界面策略为提高钒基锌金属电池的稳定性提供了宝贵的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号